Difference between revisions of "Part:BBa K3196014"
GlacierHOLE (Talk | contribs) |
GlacierHOLE (Talk | contribs) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 15: | Line 15: | ||
<h1>'''DNA Gel Electrophoretic'''</h1> | <h1>'''DNA Gel Electrophoretic'''</h1> | ||
To confirm the function of this part, first we confirm that the gene is transferred to P. pastoris GS115 successfully. | To confirm the function of this part, first we confirm that the gene is transferred to P. pastoris GS115 successfully. | ||
| + | |||
1.DNA extraction of the E.coli plasmid and verification of the right fragment. | 1.DNA extraction of the E.coli plasmid and verification of the right fragment. | ||
| + | |||
2.Prepare the competent cells of P. pastoris GS115. | 2.Prepare the competent cells of P. pastoris GS115. | ||
| + | |||
3.Electro transformation. | 3.Electro transformation. | ||
| + | |||
4.Yeast genome extraction and PCR verification. | 4.Yeast genome extraction and PCR verification. | ||
| + | |||
As the picture shows, we have constructed the engineering bacteria successfully. | As the picture shows, we have constructed the engineering bacteria successfully. | ||
[[File:T--HUST-China--2019-DNA Gel Electrophoretic.png|400px|thumb|center|Figure1:This is the DNA Gel Electrophoretic after the PCR of engineering P. pastoris GS115 genomo. ]] | [[File:T--HUST-China--2019-DNA Gel Electrophoretic.png|400px|thumb|center|Figure1:This is the DNA Gel Electrophoretic after the PCR of engineering P. pastoris GS115 genomo. ]] | ||
| Line 30: | Line 35: | ||
<h1>'''Enzyme Activity'''</h1> | <h1>'''Enzyme Activity'''</h1> | ||
Laccase activity was determined at room temperature (22–25 °C) using ABTS. Oxidation of ABTS (1 mM) was measured at 420 nm (ε = 36,000 M−1 cm−1) in 20 mM acetate buffer (pH 4.0). | Laccase activity was determined at room temperature (22–25 °C) using ABTS. Oxidation of ABTS (1 mM) was measured at 420 nm (ε = 36,000 M−1 cm−1) in 20 mM acetate buffer (pH 4.0). | ||
| − | By using this formula: | + | |
| − | We obtain the follow figure that represent the enzyme activity. | + | By using this formula:〖activity=(A2−A1)〗∕t∗11244 |
| − | + | ||
| + | We obtain the follow figure that represent the enzyme activity change with time. | ||
| + | |||
[[File:T--HUST-China--2019-PHO1 pro-SLAC.png|400px|thumb|center|Figure4:This is the engineering P. pastoris GS115(PHO1-SLAC) enzyme activity curve. ]] | [[File:T--HUST-China--2019-PHO1 pro-SLAC.png|400px|thumb|center|Figure4:This is the engineering P. pastoris GS115(PHO1-SLAC) enzyme activity curve. ]] | ||
Latest revision as of 12:06, 21 October 2019
AOX1-Kozak-PHO1 pro-SLAC-His tag-AOX1 Terminator
PHO1 αpro is a combined signal peptide, which enhance the enzyme activity 3.5 times. SLAC can catalyze lignin.
Characterization
This is a composite part that used to degraded lignin. SLAC is a multicopper oxidase isolated from S. coelicolor , capable of catalyzing one-electron oxidation of a wide range of substrates to generate radicals while concomitantly reducing molecular oxygen to water[1].
PHO1 is one of three repressible acid phosphatases, a glycoprotein that is transported to the cell surface.[2] P. pastoris is usually the preferred host for the production of industrial enzymes.
DNA Gel Electrophoretic
To confirm the function of this part, first we confirm that the gene is transferred to P. pastoris GS115 successfully.
1.DNA extraction of the E.coli plasmid and verification of the right fragment.
2.Prepare the competent cells of P. pastoris GS115.
3.Electro transformation.
4.Yeast genome extraction and PCR verification.
As the picture shows, we have constructed the engineering bacteria successfully.
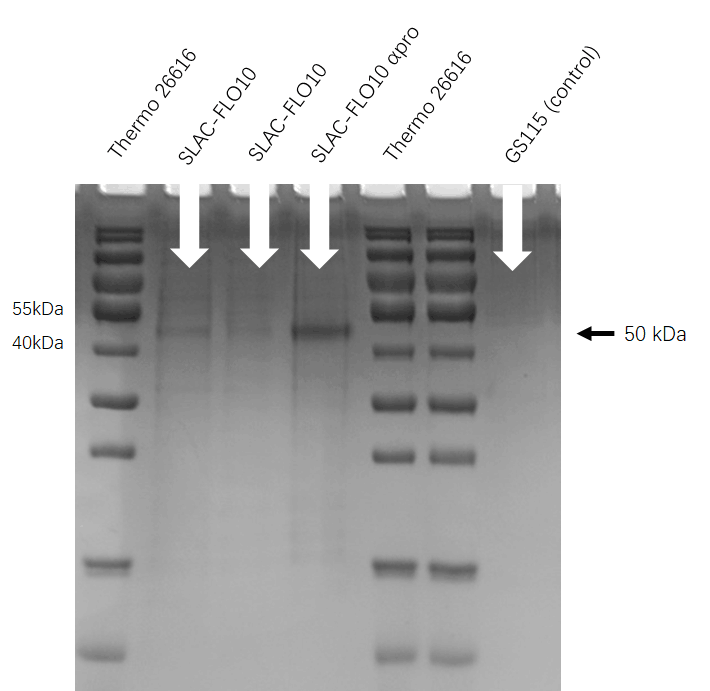
SDS-PAGE
Second, we cultured the engineering P. pastoris GS115(PHO1-SLAC)in the buffered glycerol-complex medium (BMGY) and induced it in buffered minimal methanol medium (BMM).
Enzyme Activity
Laccase activity was determined at room temperature (22–25 °C) using ABTS. Oxidation of ABTS (1 mM) was measured at 420 nm (ε = 36,000 M−1 cm−1) in 20 mM acetate buffer (pH 4.0).
By using this formula:〖activity=(A2−A1)〗∕t∗11244
We obtain the follow figure that represent the enzyme activity change with time.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 937
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1568