Difference between revisions of "Part:BBa K3196012"
GlacierHOLE (Talk | contribs) |
GlacierHOLE (Talk | contribs) |
||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K3196012 short</partinfo> | <partinfo>BBa_K3196012 short</partinfo> | ||
| + | The FLO10 was combined with the guiding peptide sequence of saccharomyces cerevisiae to form the signal peptide FLO10- αpro.FLO10 αpro is a combined signal peptide, which enhance the enzyme activity 3 times. | ||
| + | <h1>'''Characterization'''</h1> | ||
| + | This is a composite part that used to degraded lignin. | ||
| + | SLAC is a multicopper oxidase isolated from S. coelicolor , capable of catalyzing one-electron oxidation of a wide range of substrates to generate radicals while concomitantly reducing molecular oxygen to water<sup>[1]</sup>. | ||
| + | |||
| + | The signal peptide FLO10 was combined with the guiding peptide sequence of saccharomyces cerevisiae to form the signal peptide FLO10- αpro.FLO10 αpro is a combined signal peptide, which enhance the enzyme activity 3 times.<sup>[2]</sup> | ||
| + | P. pastoris is usually the preferred host for the production of industrial enzymes. | ||
| + | |||
| + | [[File:T--HUST--China--2019-FLO10-αproSLAC.jpg |400px|thumb|center|Figure1.This is the pathway of this composite part.]] | ||
| + | |||
| + | <h1>'''DNA Gel Electrophoretic'''</h1> | ||
| + | To confirm the function of this part, first we confirm that the gene is transferred to P. pastoris GS115 successfully. | ||
| + | 1.DNA extraction of the E.coli plasmid and verification of the right fragment. | ||
| + | 2.Prepare the competent cells of P. pastoris GS115. | ||
| + | 3.Electro transformation. | ||
| + | 4.Yeast genome extraction and PCR verification. | ||
| + | As the picture shows, we have constructed the engineering bacteria successfully. | ||
| + | [[File:T--HUST-China--2019-DNA Gel Electrophoretic.png|400px|thumb|center|Figure1:This is the DNA Gel Electrophoretic after the PCR of engineering P. pastoris GS115 genomo. ]] | ||
| + | |||
| + | |||
| + | <h1>'''SDS-PAGE'''</h1> | ||
| + | Second, we cultured the engineering P. pastoris GS115(FLO10 pro-SLAC)in the buffered glycerol-complex medium (BMGY) and induced it in buffered minimal methanol medium (BMM). | ||
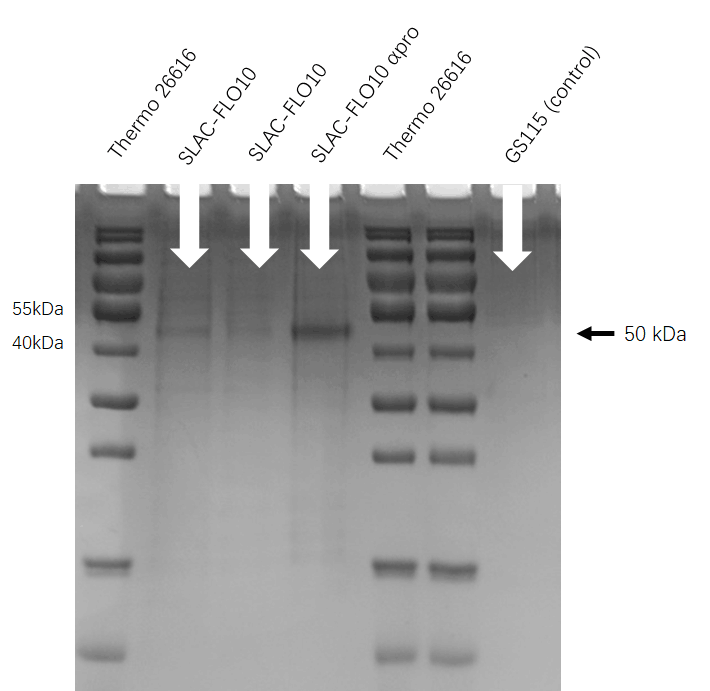
| + | [[File:T--HUST-China--2019-SLAC-SDS-PAGE.jpg |400px|thumb|center|Figure3:The SDS-page result shows that engineering P. pastoris GS115(FLO10 pro-SLAC) have successfully secrete SLAC protein into superfluous liquid.]] | ||
| + | |||
| + | |||
| + | <h1>'''Enzyme Activity'''</h1> | ||
| + | Laccase activity was determined at room temperature (22–25 °C) using ABTS. Oxidation of ABTS (1 mM) was measured at 420 nm (ε = 36,000 M−1 cm−1) in 20 mM acetate buffer (pH 4.0). | ||
| + | By using this formula: | ||
| + | 〖activity=(A2−A1)〗∕t∗11244 | ||
| + | We obtain the follow figure that represent the enzyme activity changes with time. | ||
| + | As the figure shows, which confirm the enzyme activity. | ||
| + | [[File:T--HUST-China--2019-FLO10 pro-SLAC.png|400px|thumb|center|Figure4:This is the engineering P. pastoris GS115(FLO10 pro-SLAC) enzyme activity curve. ]] | ||
<!-- --> | <!-- --> | ||
| Line 12: | Line 44: | ||
<partinfo>BBa_K3196012 parameters</partinfo> | <partinfo>BBa_K3196012 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 09:45, 21 October 2019
AOX1-Kozak-FLO10 pro-SLAC-His tag-AOX1 Terminator
The FLO10 was combined with the guiding peptide sequence of saccharomyces cerevisiae to form the signal peptide FLO10- αpro.FLO10 αpro is a combined signal peptide, which enhance the enzyme activity 3 times.
Characterization
This is a composite part that used to degraded lignin. SLAC is a multicopper oxidase isolated from S. coelicolor , capable of catalyzing one-electron oxidation of a wide range of substrates to generate radicals while concomitantly reducing molecular oxygen to water[1].
The signal peptide FLO10 was combined with the guiding peptide sequence of saccharomyces cerevisiae to form the signal peptide FLO10- αpro.FLO10 αpro is a combined signal peptide, which enhance the enzyme activity 3 times.[2] P. pastoris is usually the preferred host for the production of industrial enzymes.
DNA Gel Electrophoretic
To confirm the function of this part, first we confirm that the gene is transferred to P. pastoris GS115 successfully. 1.DNA extraction of the E.coli plasmid and verification of the right fragment. 2.Prepare the competent cells of P. pastoris GS115. 3.Electro transformation. 4.Yeast genome extraction and PCR verification. As the picture shows, we have constructed the engineering bacteria successfully.
SDS-PAGE
Second, we cultured the engineering P. pastoris GS115(FLO10 pro-SLAC)in the buffered glycerol-complex medium (BMGY) and induced it in buffered minimal methanol medium (BMM).
Enzyme Activity
Laccase activity was determined at room temperature (22–25 °C) using ABTS. Oxidation of ABTS (1 mM) was measured at 420 nm (ε = 36,000 M−1 cm−1) in 20 mM acetate buffer (pH 4.0). By using this formula: 〖activity=(A2−A1)〗∕t∗11244 We obtain the follow figure that represent the enzyme activity changes with time. As the figure shows, which confirm the enzyme activity.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 937
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1586