Difference between revisions of "Part:BBa K346004"
JjunyiJiao (Talk | contribs) |
|||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K346004 short</partinfo> | <partinfo>BBa_K346004 short</partinfo> | ||
| − | |||
| + | This part was designed to function as lead binding peptide in our project. It was designed to be expressed in the cytosol but when fused with the periplasm protein DsbA and membrane protein Lpp-OmpA, it will be expressed and translocated to the periplasm and membrane, respectively. In short, this part functions as a basic lead binding peptide part in our project. | ||
===Description=== | ===Description=== | ||
| Line 10: | Line 10: | ||
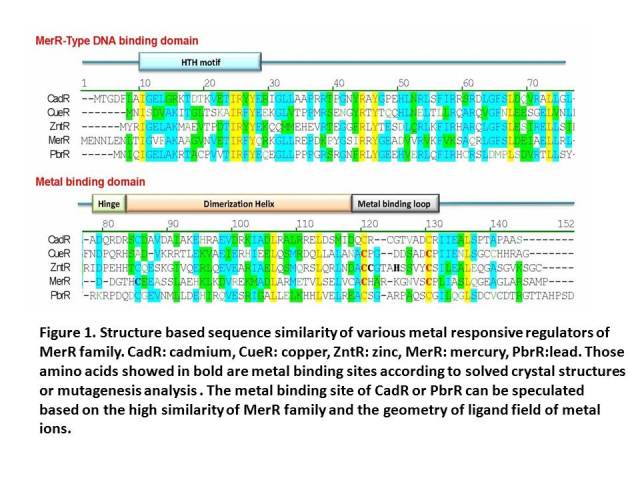
| − | MerR family TFs share a high similarity at the C-terminal metal binding domain , which indicates a similar metal recognition mechanism and metal-protein complex structure.Previous work shows that C-terminal metal binding domain can act as a metal accumulator without the help of the N-terminal DNA binding domain. | + | MerR family TFs share a high similarity at the C-terminal metal binding domain, which indicates a similar metal recognition mechanism and metal-protein complex structure. Previous work shows that C-terminal metal binding domain can act as a metal accumulator without the help of the N-terminal DNA binding domain. Function test of mercury MBP showed that our whole-cell bioabsorbents can absorb more than 50% of 10-6 M Hg (II) in 120 minutes, indicating that cells expressing certain metal binding peptide engineered via our method can decontaminate corresponding metal ion from aquatic environment. This design has been successfully applied to another homolog of MerR family – PbrR, the lead responsive regulator. In our project, we tried to tandem two copies of metal binding domains together to make a high performance and less energy consuming metal binding peptide. Our bacteria achieved an equivalent lead accumulation capacity as the mercury MBP, which proved validness of our engineering strategy. This part worked as the core part in the lead bioabsorption device. |
| − | [[Image:lead | + | [[Image:PbrR.jpg]] [[Image:lead MBP1.jpg]] |
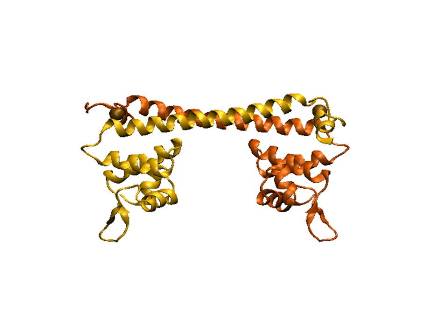
| − | The figure shows the structure | + | The figure shows the structure of PbrR and MBP(lead). The left is the structure of PbrR, a member of MerR family and the right shows the predicted structure of resulted metal binding peptide, which was engineered from PbrR. Lead ions are indicated as black balls in metal binding pockets. |
Latest revision as of 09:42, 21 October 2019
RBS(B0034)_MBP(lead metal binding peptide egineered from PbrR)+Terminator(B0015)
This part was designed to function as lead binding peptide in our project. It was designed to be expressed in the cytosol but when fused with the periplasm protein DsbA and membrane protein Lpp-OmpA, it will be expressed and translocated to the periplasm and membrane, respectively. In short, this part functions as a basic lead binding peptide part in our project.
Description
MerR family TFs share a high similarity at the C-terminal metal binding domain, which indicates a similar metal recognition mechanism and metal-protein complex structure. Previous work shows that C-terminal metal binding domain can act as a metal accumulator without the help of the N-terminal DNA binding domain. Function test of mercury MBP showed that our whole-cell bioabsorbents can absorb more than 50% of 10-6 M Hg (II) in 120 minutes, indicating that cells expressing certain metal binding peptide engineered via our method can decontaminate corresponding metal ion from aquatic environment. This design has been successfully applied to another homolog of MerR family – PbrR, the lead responsive regulator. In our project, we tried to tandem two copies of metal binding domains together to make a high performance and less energy consuming metal binding peptide. Our bacteria achieved an equivalent lead accumulation capacity as the mercury MBP, which proved validness of our engineering strategy. This part worked as the core part in the lead bioabsorption device.
The figure shows the structure of PbrR and MBP(lead). The left is the structure of PbrR, a member of MerR family and the right shows the predicted structure of resulted metal binding peptide, which was engineered from PbrR. Lead ions are indicated as black balls in metal binding pockets.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]