Difference between revisions of "Part:BBa K1033916:Experience"
Ethanagena (Talk | contribs) (→User Reviews) |
|||
| (11 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
===Applications of BBa_K1033916=== | ===Applications of BBa_K1033916=== | ||
| − | |||
===User Reviews=== | ===User Reviews=== | ||
<!-- DON'T DELETE --><partinfo>BBa_K1033916 StartReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_K1033916 StartReviews</partinfo> | ||
| Line 26: | Line 25: | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
<b> Fluorescent properties of amajLime </b> | <b> Fluorescent properties of amajLime </b> | ||
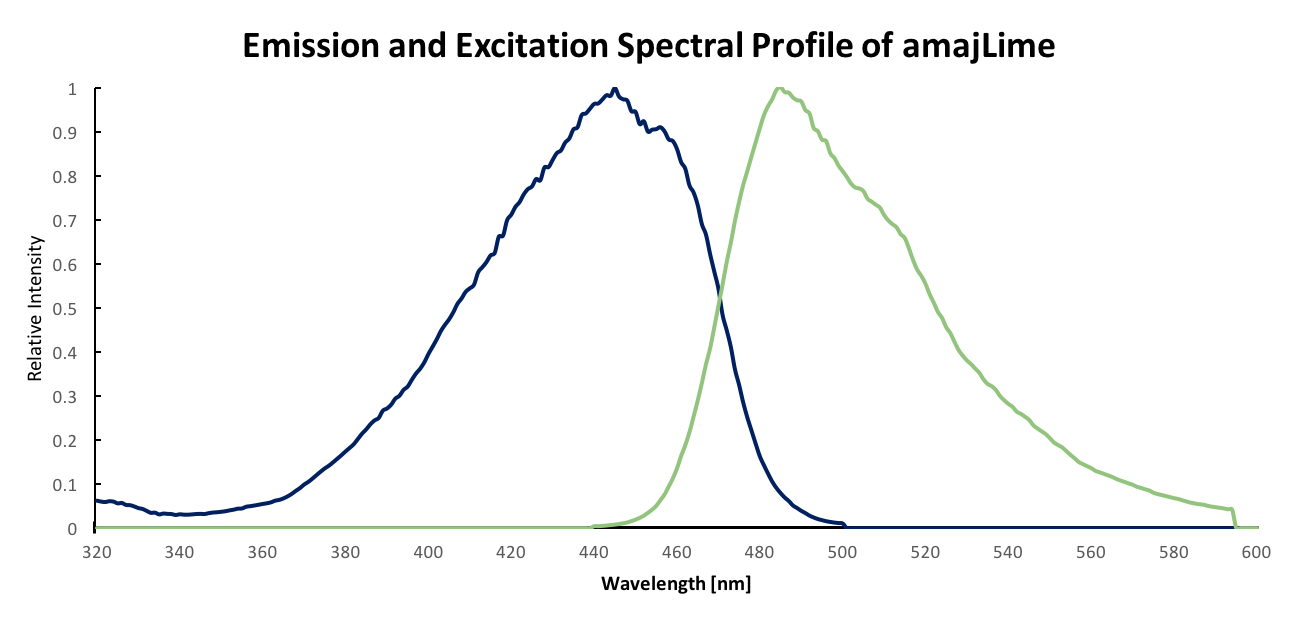
| − | <p> | + | <p> Although amajLime is described as chromoprotein in main page, we characterised its spectral properties and found the max excitation and emission wavelength at 445 nm nd 485 nm respectively.</p> |
| − | [[File:Ex-Em. amajlime.png|width='60%' valign='top'| |center|thumb|550px|''<b>Fig.1</b> Emission and Excitation spectra]] | + | [[File:Ex-Em. amajlime.png|width='60%' valign='top'| |center|thumb|550px|''<b>Fig.1</b> Emission and Excitation spectra in blue and green respectively.]] |
<br> | <br> | ||
<b> Charaterization of pH stability of amajLime </b> | <b> Charaterization of pH stability of amajLime </b> | ||
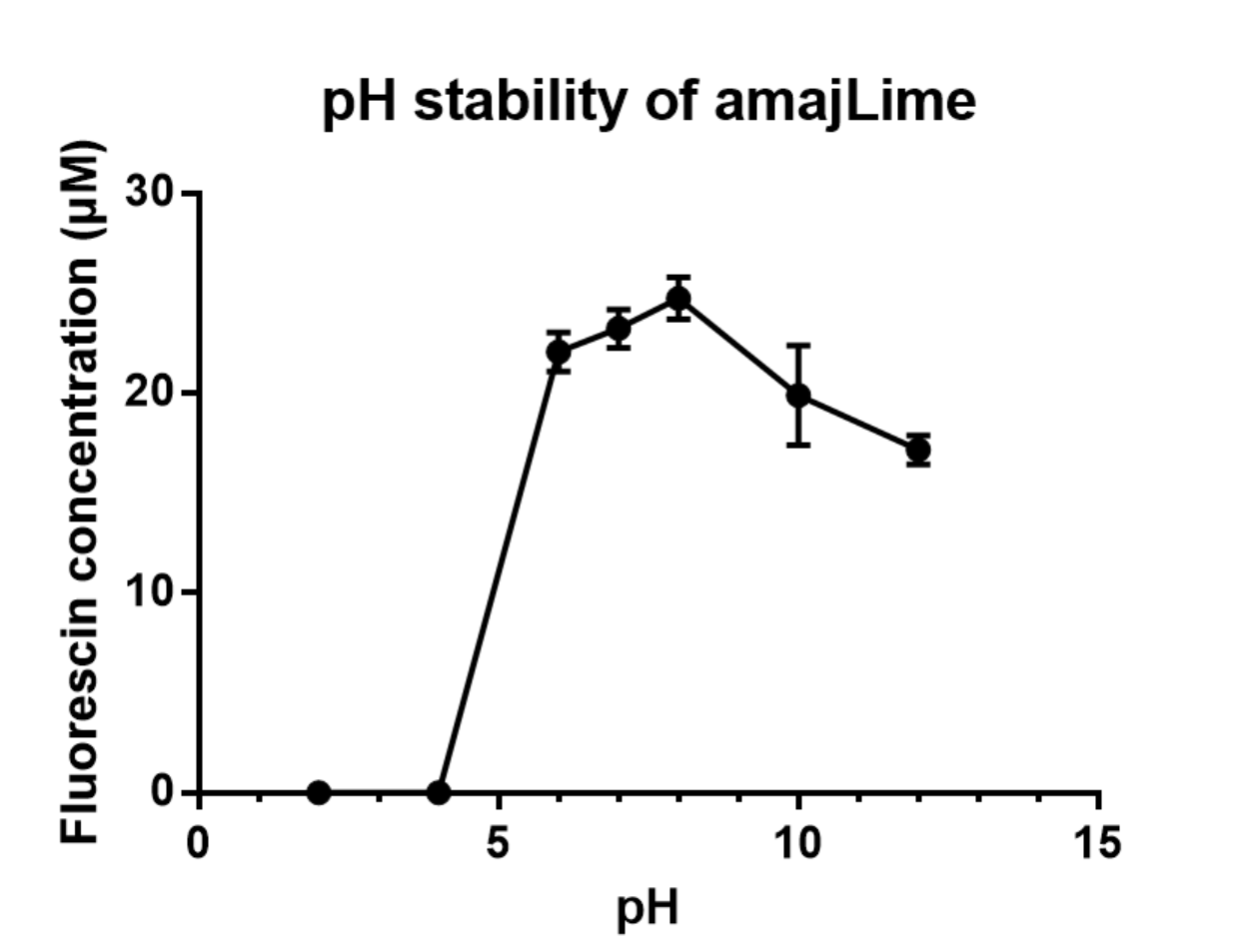
| − | <p>We | + | <p>We transformed part BBa_K1033916 with constituitive promoter: J23100 in C41 and grew in 2XYT for 24 hours. Purifying the amajLime by Ion Exchange Chromatography and Hydrophobic Interaction Chromatography, we measured the fluoresece of purified amajLime, which is diluted to 10µg/100µl (total 200µl) in triplicates, in different buffers (ranges from pH2 to pH12). The result shows that the stability drops dramatically in pH condition below 6 and attains maxima fluorescence at pH 8.</p> |
<div align="center"> | <div align="center"> | ||
| − | [[File:Amaj.PNG |center|thumb|350px|''<b>Fig.2</b> Vary pH attributed to different fluorescent intensity of | + | [[File:Amaj.PNG |center|thumb|350px|''<b>Fig.2</b> Vary pH attributed to different fluorescent intensity of amajLime.]] |
<table cellpadding="2" border="1px" cellspacing="0" align="center" width="70%"> | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="70%"> | ||
| − | < | + | |
| − | + | <td><b>Measurement Type</b></td> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<td>Fluorescence</td> | <td>Fluorescence</td> | ||
| − | + | ||
<tr> | <tr> | ||
| − | <td>Microplate name</td> | + | <td><b>Microplate name</b></td> |
<td>COSTAR 96</td> | <td>COSTAR 96</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Scan mode</td> | + | <td><b>Scan mode</b></td> |
<td>orbital averaging</td> | <td>orbital averaging</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Scan diameter [nm]</td> | + | <td><b>Scan diameter [nm]</b></td> |
<td>3</td> | <td>3</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Excitation</td> | + | <td><b>Excitation</b></td> |
<td>470-15</td> | <td>470-15</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Emission</td> | + | <td><b>Emission</b></td> |
<td>515-20</td> | <td>515-20</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Dichronic filter </td> | + | <td><b>Dichronic filter </b></td> |
<td>auto 491.2</td> | <td>auto 491.2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Gain </td> | + | <td><b>Gain </b></td> |
<td>500</td> | <td>500</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Focal height [nm]</td> | + | <td><b>Focal height [nm]</b></td> |
<td>9</td> | <td>9</td> | ||
</tr> | </tr> | ||
| + | <caption><p align="justify"><b>Table 1</b> Plate reader setting of fluorescent measurement</p></caption> | ||
</table> | </table> | ||
|}; | |}; | ||
Latest revision as of 06:41, 21 October 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1033916
User Reviews
UNIQ34747994e75a4c22-partinfo-00000000-QINU UNIQ34747994e75a4c22-partinfo-00000001-QINU
|
•••••
Hong Kong-CUHK iGEM 2017 |
Fluorescent properties of amajLime Although amajLime is described as chromoprotein in main page, we characterised its spectral properties and found the max excitation and emission wavelength at 445 nm nd 485 nm respectively.
We transformed part BBa_K1033916 with constituitive promoter: J23100 in C41 and grew in 2XYT for 24 hours. Purifying the amajLime by Ion Exchange Chromatography and Hydrophobic Interaction Chromatography, we measured the fluoresece of purified amajLime, which is diluted to 10µg/100µl (total 200µl) in triplicates, in different buffers (ranges from pH2 to pH12). The result shows that the stability drops dramatically in pH condition below 6 and attains maxima fluorescence at pH 8.
|