Difference between revisions of "Part:BBa T9002"
| Line 23: | Line 23: | ||
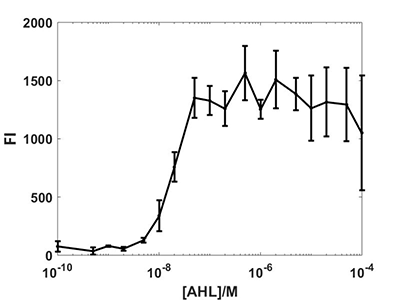
We retest the characterization of this part.Using different concentration of AHL,we get the corresponding FI and the result is as follows. | We retest the characterization of this part.Using different concentration of AHL,we get the corresponding FI and the result is as follows. | ||
<center>https://2019.igem.org/wiki/images/c/cb/T--Peking--QS2.png</center> | <center>https://2019.igem.org/wiki/images/c/cb/T--Peking--QS2.png</center> | ||
| − | Figure1 The corresponding FI of different concentration of AHL.<br> | + | <center>Figure1 The corresponding FI of different concentration of AHL.</center><br> |
Experiment procedure:<br> | Experiment procedure:<br> | ||
1.E. coli DH5α transferred with PSB1C3-T9002 were incubated in LB liquid medium at 37℃, 220rpm overnight<br> | 1.E. coli DH5α transferred with PSB1C3-T9002 were incubated in LB liquid medium at 37℃, 220rpm overnight<br> | ||
| Line 33: | Line 33: | ||
We used part T9002 to characterizing quorum sensing system. But we found that this part has an obvious homologous recombination. So we changed the terminator of GFP to avoid it. | We used part T9002 to characterizing quorum sensing system. But we found that this part has an obvious homologous recombination. So we changed the terminator of GFP to avoid it. | ||
<center>https://2019.igem.org/wiki/images/e/e2/T--Peking--QS.png</center> | <center>https://2019.igem.org/wiki/images/e/e2/T--Peking--QS.png</center> | ||
| − | Figure2 The design of quorum sensing system | + | <center>Figure2 The design of quorum sensing system</center> |
<center>https://2019.igem.org/wiki/images/1/10/T--Peking--QS1.png</center> | <center>https://2019.igem.org/wiki/images/1/10/T--Peking--QS1.png</center> | ||
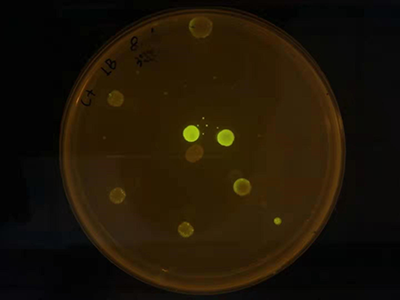
| − | Figure3 We use this part of a AHL receiver. With the distance between donor and receiver cells, the GFP expression level is significantly changed. | + | <center>Figure3 We use this part of a AHL receiver. With the distance between donor and receiver cells, the GFP expression level is significantly changed.</center> |
| − | Experiment procedure: | + | Experiment procedure:<br> |
| − | 1.Incubate donor and receiver cells in LB medium overnight. | + | 1.Incubate donor and receiver cells in LB medium overnight.<br> |
| − | 2.Grown culture was 100-fold diluted in M9 medium. | + | 2.Grown culture was 100-fold diluted in M9 medium. <br> |
| − | 3.5 μL donor cell was dropped in the LB solid medium. | + | 3.5 μL donor cell was dropped in the LB solid medium. <br> |
| − | 4.5 Μl receptor cell was dropped in different distances in the same LB solid medium | + | 4.5 Μl receptor cell was dropped in different distances in the same LB solid medium.<br> |
Revision as of 22:30, 20 October 2019
GFP Producer Controlled by 3OC6HSL Receiver Device
The luxR based receiver, F2620 (formerly I13270), controls the production of GFP. The GFP protein generator is the same as that found in I7101.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1004
Illegal BsaI.rc site found at 1732
GFP Producer Controlled by 3OC6HSL Receiver Device
(Characterized by Peking_2019)
We retest the characterization of this part.Using different concentration of AHL,we get the corresponding FI and the result is as follows.
Experiment procedure:
1.E. coli DH5α transferred with PSB1C3-T9002 were incubated in LB liquid medium at 37℃, 220rpm overnight
2.Grown culture was 100-fold diluted in M9 medium for the same condition for 3 hour.
3.Culture was 40-fold diluted in M9 medium containing different concentration of AHL.
4.200 µl aliquots of each of the cultures were transferred into a flat-bottomed 96 well plate.
5.FI and OD600 was measured for 10 hours in microplate reader.
We used part T9002 to characterizing quorum sensing system. But we found that this part has an obvious homologous recombination. So we changed the terminator of GFP to avoid it.

Experiment procedure:
1.Incubate donor and receiver cells in LB medium overnight.
2.Grown culture was 100-fold diluted in M9 medium.
3.5 μL donor cell was dropped in the LB solid medium.
4.5 Μl receptor cell was dropped in different distances in the same LB solid medium.
