Difference between revisions of "Part:BBa K1033933:Experience"
(→Applications of BBa_K1033933) |
(→Applications of BBa_K1033933) |
||
| (41 intermediate revisions by 3 users not shown) | |||
| Line 8: | Line 8: | ||
2019 iGEM team Linkoping Sweden validated this part.<br><br> | 2019 iGEM team Linkoping Sweden validated this part.<br><br> | ||
| + | <html> | ||
| + | |||
| + | Summary: We studied the expression in of <I>E. coli</I> BL21(DE3) containing CBD-asPink (<partinfo>BBa_K3182100</partinfo>) protein with a constitutive promotor to see if the protein could be used for a pink/white screening. We also tested the bindning capacity of the CBD-asPink protein to a micro cellulose bandage and then studied the release mechanism. | ||
<br><br> | <br><br> | ||
| − | |||
| − | |||
| − | |||
| + | <b> Pink-white screening </b><br> | ||
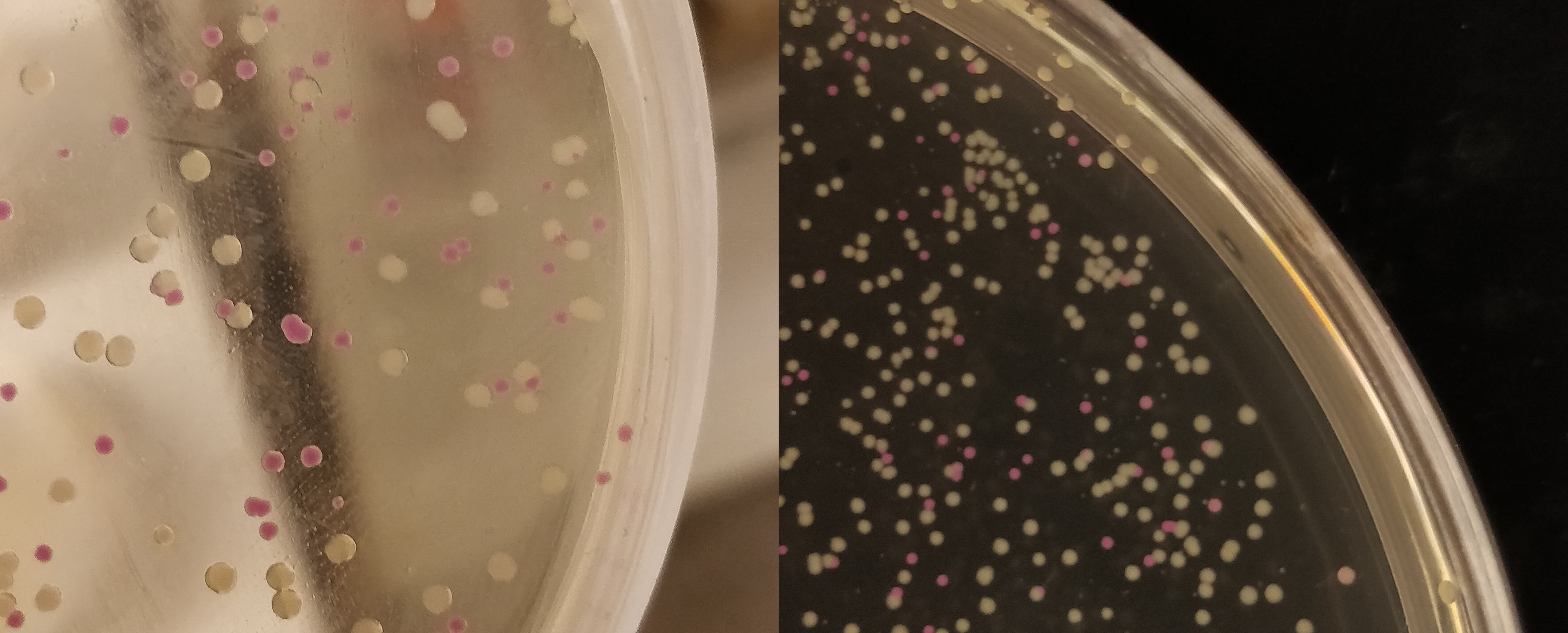
| + | To visualize the absorbance of AsPink on a petri dish, BL21 (DE3) colonies containing the pUC19-pCons-AsPink part (<a href="https://parts.igem.org/Part:BBa_K3182100">BBa_K3182100</a>), which was used in the pink-white screening, were incubated for 16 hours in 37 °C. The pCons-AsPink construct was removed and (Magainin 2) and <a href="https://parts.igem.org/Part:BBa_K3182104 ">BBa_K3182104</a> (CHAP) was inserted into the vector. The pink colonies contain AsPink and indicate a religated pCons-AsPink. The white colonies indicate a successful ligation. Below (Figure 1) is a picture of the pink-white screening. | ||
| + | |||
| + | </html> | ||
| + | [[File:T--Linkoping_Sweden--asPinkcompilation.png|350px|thumb|left|<b>Figure 2. </b><u>A:</u> E. coli BL21 cells expressing the biobrick, incubated for 16 hours at 16°C at 80 rpm in 1L of LB-miller. <u>B:</u> Lysated (via sonication) BL21s which are expressing the biobrick. It is lysate from the culture above in figure1. Incubated for 16 hours at 16°C at 80 rpm in 1 litre of LB-miller. <u>C:</u>Centrifuged lysate of BL21 culture which express the biobrick. It is the same culture as figure 4 and 5. A pellet of non-lysated bacteria can be observed.]]<html> | ||
| + | |||
| + | </html>[[File:T--Linkoping_Sweden--pinkwhite156.jpeg|510px|thumb|right|<b>Figure 1.</b> | ||
| + | BL21 (DE3) bacteria used for pink-white screening. The white colonies indicate a successful ligation and the pink colonies is AsPink expressing bacteria which signals a false-positive ligation. ]]<html> | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | |||
| + | <br><br><br><br> | ||
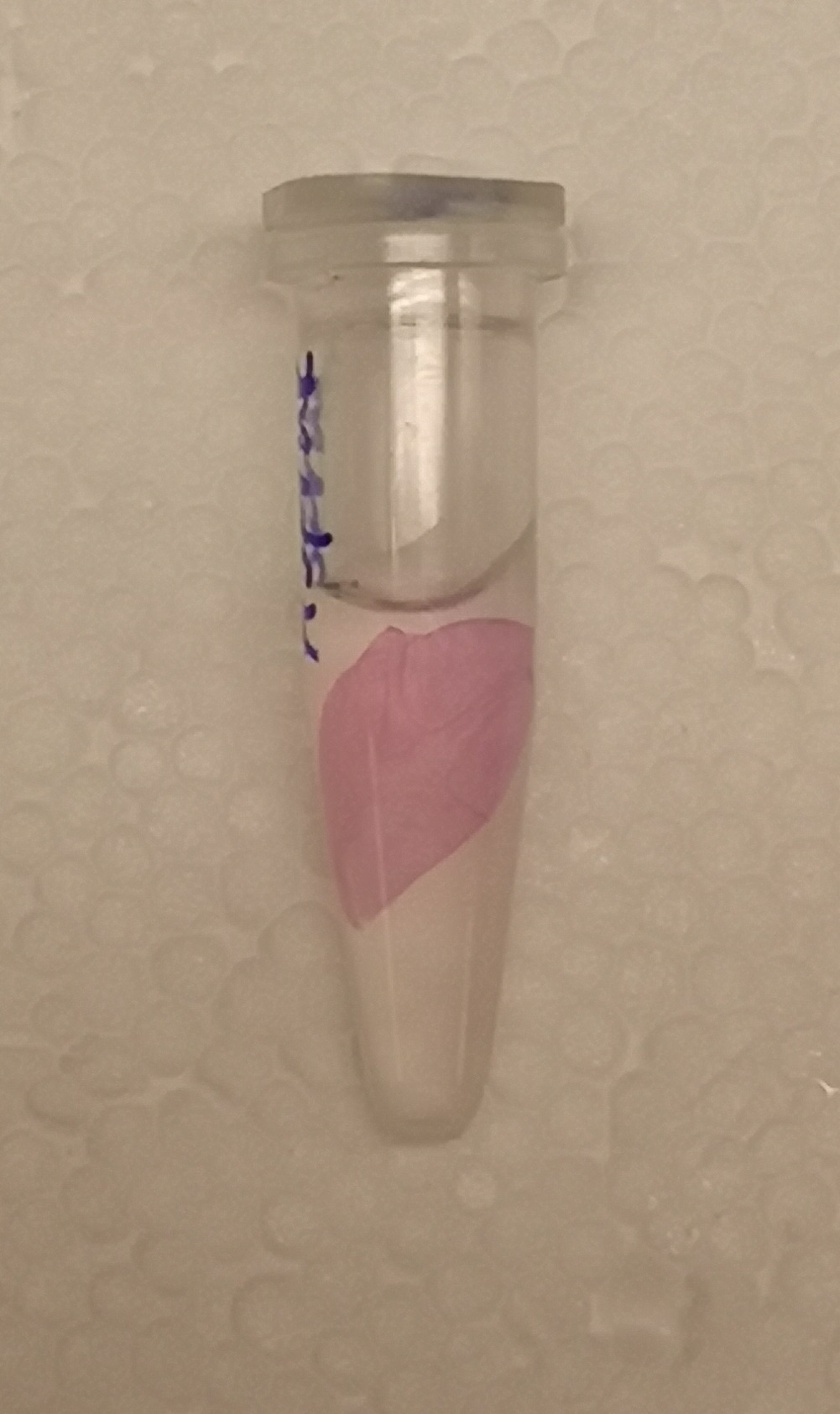
| + | <b>CBD-asPink bindning capacity</b><br> | ||
| + | </html>[[File:T--Linkoping Sweden--aspink bunden.jpeg|250px|right|thumb|<b><I>Figure 3.</I></b> Lysate (via sonication) from <I>E. coli</I> BL21 was incubated with Epiprotect (microbial cellulose bandage) for 1h and washed thrice with 70% ethanol. ]]<html> | ||
| + | To test the bindning capacity of CBD-asPink (<partinfo>BBa_K3182000</partinfo>) the microbial cellulose bandage was suspended into sonicated lysate of <I>E.coli</I> BL21(DE3) with the expressed fusion protein (<b><I>Figure 3</I></b>) and incubated for 30 minutes. | ||
| + | The bandage was then washed thrice in 70% ethanol which confirmed that the bindning of CDB-asPink was specific for the cellulose bandage. | ||
<br><br> | <br><br> | ||
| − | [[File:T--Linkoping Sweden--aspink bunden vs obunden.jpeg| | + | </html>[[File:T--Linkoping Sweden--aspink bunden vs obunden.jpeg|250px|left|thumb|<b><I>Figure 4.</I></b> To test the release mechanism of CBD-asPink, human thrombin and cleavage buffer was added to the bandage and incubated for 16 hours on an end to end rotator. The figure is the result after the incubation with a negative control with only cleavage buffer (left) and human thrombin and cleavage buffer (right). ]]<html> <b> CBD-asPink with thrombin cleavage</b><br> |
| − | < | + | |
| − | + | After the washes, human thrombin and cleavage buffer was added to the bandage with bound CBD-asPink to test the release mechanism of the fusion protein. The bandage was then incubated with the solution for 16 hours on an end to end rotator together with an negative control containing cellulose bandage and only cleavage buffer. | |
| − | < | + | After the incubation, the supernatant containing thrombin was pink while the negative control containing only cleavage buffer was transparent with a clear pink cellulose bandage (<b><I>Figure 4</I></b>). This indicates that the release mechanism work and the AsPink protein has successfully been released from the bandage into the supernatant. |
| − | < | + | <html> |
| − | < | + | |
<br><br> | <br><br> | ||
| + | </html> | ||
| + | |||
| + | <br> | ||
<br><br> | <br><br> | ||
<br><br> | <br><br> | ||
Latest revision as of 16:59, 20 October 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1033933
2019 iGEM team Linkoping Sweden
2019 iGEM team Linkoping Sweden validated this part.
Summary: We studied the expression in of E. coli BL21(DE3) containing CBD-asPink (
Pink-white screening
To visualize the absorbance of AsPink on a petri dish, BL21 (DE3) colonies containing the pUC19-pCons-AsPink part (BBa_K3182100), which was used in the pink-white screening, were incubated for 16 hours in 37 °C. The pCons-AsPink construct was removed and (Magainin 2) and BBa_K3182104 (CHAP) was inserted into the vector. The pink colonies contain AsPink and indicate a religated pCons-AsPink. The white colonies indicate a successful ligation. Below (Figure 1) is a picture of the pink-white screening.

CBD-asPink bindning capacity
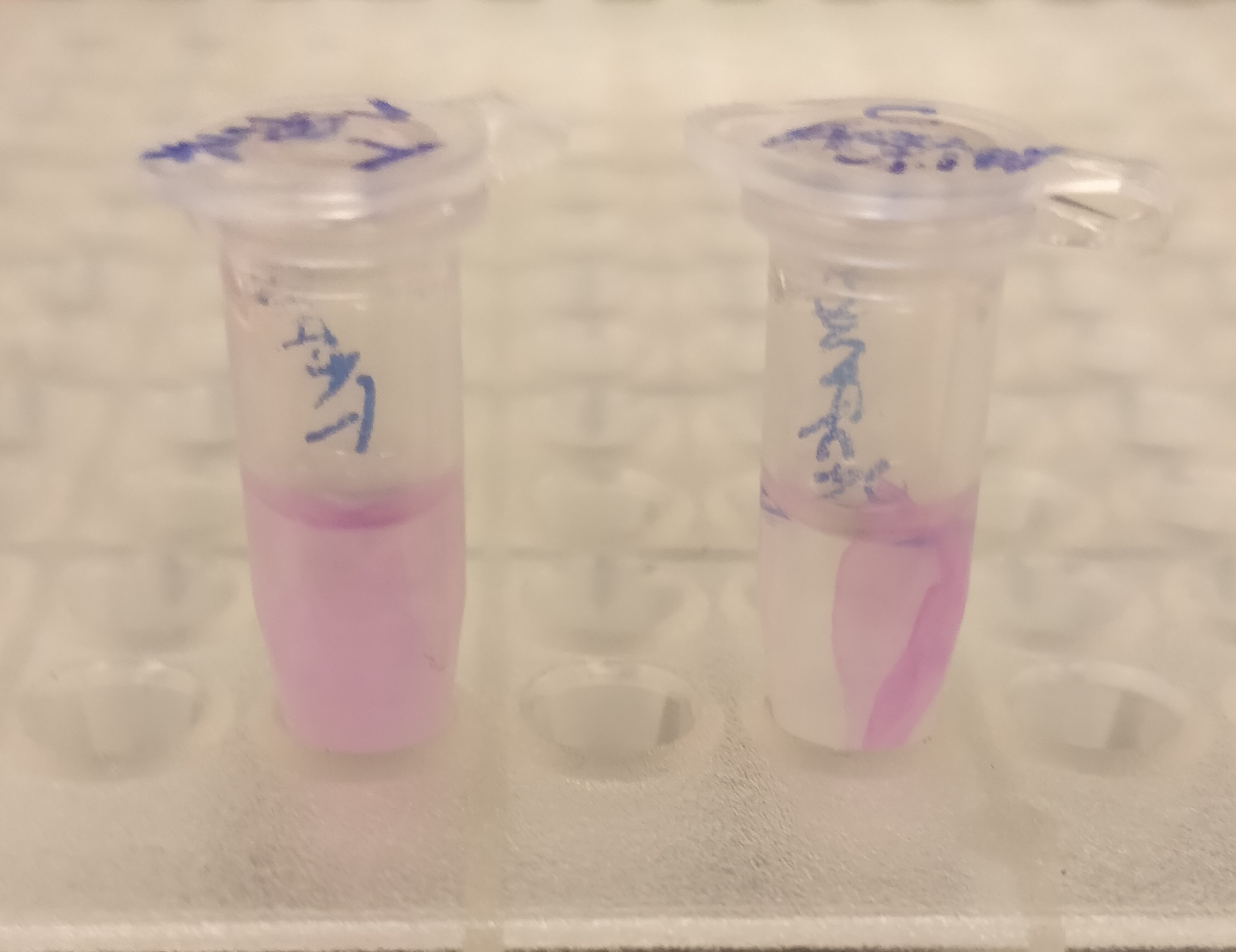
After the washes, human thrombin and cleavage buffer was added to the bandage with bound CBD-asPink to test the release mechanism of the fusion protein. The bandage was then incubated with the solution for 16 hours on an end to end rotator together with an negative control containing cellulose bandage and only cleavage buffer. After the incubation, the supernatant containing thrombin was pink while the negative control containing only cleavage buffer was transparent with a clear pink cellulose bandage (Figure 4). This indicates that the release mechanism work and the AsPink protein has successfully been released from the bandage into the supernatant.
User Reviews
UNIQ505afb2ca2738a70-partinfo-00000005-QINU UNIQ505afb2ca2738a70-partinfo-00000006-QINU