Difference between revisions of "Part:BBa I746916:Experience"
(→Applications of BBa_I746916) |
(→Applications of BBa_I746916) |
||
| Line 8: | Line 8: | ||
<h2> 2019 iGEM team Linkoping Sweden </h2> | <h2> 2019 iGEM team Linkoping Sweden </h2> | ||
| − | [[Image:T--Linkoping_Sweden--CBD-sfGFPstorodl4.jpeg|300px|thumb|left|<b>< | + | The expression system used contained the following parts: |
| + | <ul> | ||
| + | <li> </html>[https://parts.igem.org/wiki/index.php?title=Part:BBa_B0034 BBa_B0034]Ribosome binding site<html></li> | ||
| + | <li> </html>[http://https://parts.igem.org/Part:BBa_I719005 BBa_I719005]T7 promotor<html></li> | ||
| + | <br> | ||
| + | <b style=font-size:120%;> Fluorescence in BL21 (DE3)</b> <br> | ||
| + | To verify the fluorescence of sfGFP (<a href="https://parts.igem.org/Part:BBa_I746916">BBa_I746916</a>), BL21 (DE3) containing CBD-sfGFP was grown in 1 liter LB-miller with 25 µg/ml chloramphenicol. Isopropyl β-d-1-thiogalactopyranoside (IPTG) was used to induce the culture at a final concentration of 1 mM and the culture was incubated O.N. in 37 °C after the induction. Thereafter, the CBD-sfGFP expressing bacteria was placed on an UV-table emitting light 302 nm (Figure 5). The picture shows CBD-sfGFP´s strong fluorescence at 302 nm UV-light. | ||
| + | </div> | ||
| + | <br> | ||
| + | </html>[[Image:T--Linkoping_Sweden--CBD-sfGFPstorodl4.jpeg|300px|thumb|left|<b>Figure 5.</b> 1 liter LB-miller with induced BL21 (DE3) expressing CBD-sfGFP on an UV-table emitting UV-light at 302 nm.]] <html> | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | <b style=font-size:120%;> Compatibility in <i> Vibrio natriegens </i><br> </b> | ||
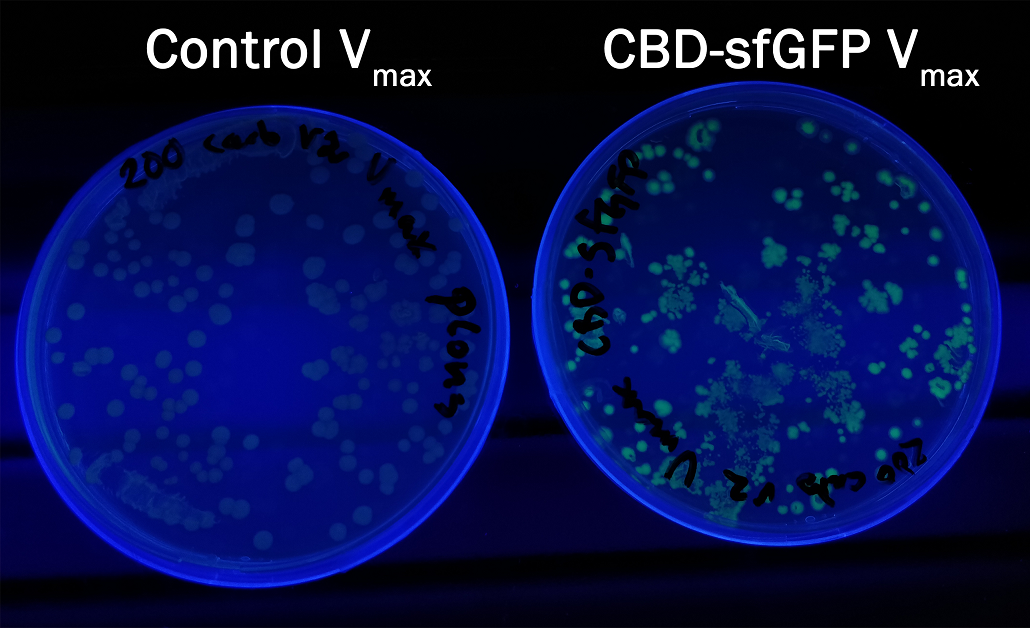
| + | In order to see if sfGFP worked in <i>Vibrio natriegens</i> using the strain V<sub>max</sub>, CBD-sfGFP (<a href="https://parts.igem.org/Part:BBa_K3182108">BBa_K3182108</a>) and CBD-pCons-Aspink (<a href="https://parts.igem.org/Part:BBa_K3182100">BBa_K3182100</a>) was ligated into the pUC19 vector and heat shocked into V<sub>max</sub>.Thereafter, the bacteria was spread onto LB-miller V2 agar dishes with 200 µg/ml carbenicillin and incubated in 37 °C for 16 hours. Both plates was put on an UV-table and illuminated in 302 nm (Figure 6). The picture below shows that the CBD-sfGFP bacteria, in comparison to the control CBD-pCons-AsPink, displays a strong green fluorescent color which verified that pUC19-CBD-sfGFP could successfully be heat shocked and expressed in V<sub>max</sub>. | ||
| − | <b>Figure | + | <div> |
| − | + | </html>[[Image:T--Linkoping_Sweden--CBD-sfGFPvibrio.jpeg|600px|thumb|left|<b>Figure 6.</b> Picture to the right depicts a LB-agar dish with<i>V<sub>maz</sub></i> expressing pUC19 CBD-sfGFP. To the left is a control with V<sub>max</sub> expressing PUC19 CBD-pCons-Aspink. Both dishes was placed on an UV-table and illuminated in 302 nm.]]<html> | |
| − | + | </div> | |
| − | + | <br><br> | |
| − | + | <br><br><br><br><br><br><br><br><br><br> | |
| − | + | <br><br><br><br><br><br><br><br><br><br> | |
| − | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | + | <br><br><br> |
| − | + | <div> | |
| − | + | <b style=font-size:120%;> Protein expression in different chassis</b><br> | |
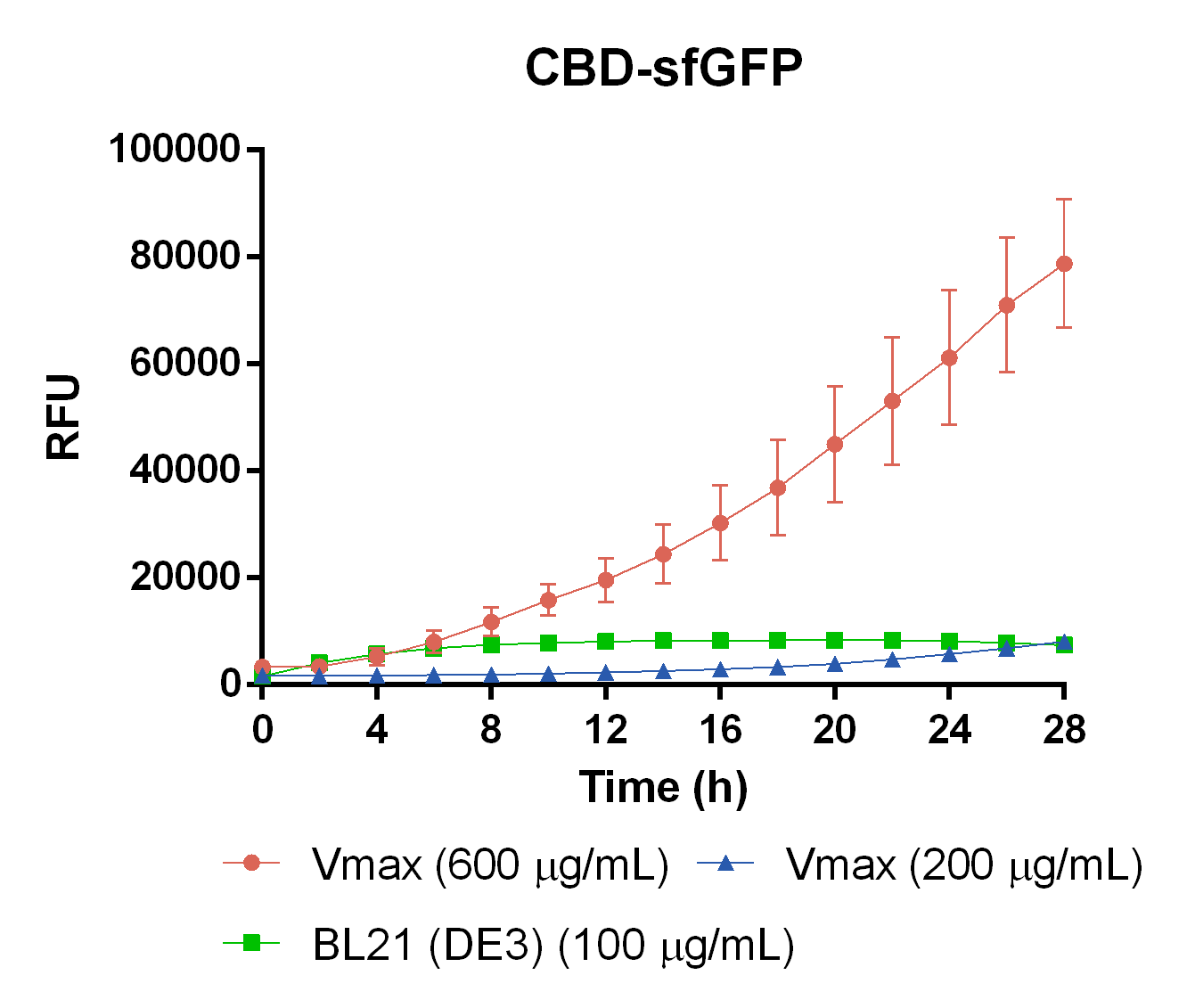
| − | <br> | + | To measure the protein expression of T7-CBD-sfGFP in different bacteria and carbenicillin concentrations. BL21 (DE3)</i> and <i> Vibrio natriegens </i>, using the strain V<sub>max</sub>, was grown in Falcon tubes to 0.5 OD<sub>600</sub>. V<sub>max</sub> was grown with two different carbenicillin concentrations, 200 and 600 µg/mL, while BL21 (DE3) had the same carbenicillin concentration of 100 µg/mL carbenicillin. The bacteria was induced with 1 mM IPTG and placed in a 96-well plate in 4 replicates with 200 µL per well. A spectrometry experiment was conducted and measured the fluorescence (excitation 470 nm,emission 550 nm) during 16 hours in 37 °C. The results seen below (Figure 7) shows that expression in V<sub>max</sub> with 600 µg/mL carbenicillin gave the highest protein yield. The most probable explanation for the increased protein yield for V<sub>max</sub> at 600 µg/mL carbenicillin is partially caused by the higher protein production of V<sub>max</sub> compared to BL21 (DE3). Another important factor was the use of an optimal concentration of carbenicillin (600 µg/mL) for V<sub>max</sub>which retained the plasmid more efficiantly than V<sub>max</sub> at 200 µg/mL carbenicillin. </div> |
| + | </html>[[Image:T--Linkoping_Sweden--CBD-VmaxEcoliMeasurement.png|600px|thumb|left|<b>Figure 7.</b> CBD-sfGFP expression in different chassis. The orange line represent Vmax at 600 µg/mL carbenicillin, blue represents Vmax at 200 µg/mL carbenicillin, and green represents E. coli BL21 at 100 µg/mL carbenicillin. The y-axis depicts RFU and the x-axis represents the time over 28 hours.]]<html> | ||
| + | </div> | ||
| + | <br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br><br><br><br><br><br><br> | ||
| + | </html> | ||
Revision as of 16:43, 20 October 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_I746916
2019 iGEM team Linkoping Sweden
The expression system used contained the following parts:
- </html>BBa_B0034Ribosome binding site
- [http://https://parts.igem.org/Part:BBa_I719005 BBa_I719005]T7 promotor
Fluorescence in BL21 (DE3)
To verify the fluorescence of sfGFP (BBa_I746916), BL21 (DE3) containing CBD-sfGFP was grown in 1 liter LB-miller with 25 µg/ml chloramphenicol. Isopropyl β-d-1-thiogalactopyranoside (IPTG) was used to induce the culture at a final concentration of 1 mM and the culture was incubated O.N. in 37 °C after the induction. Thereafter, the CBD-sfGFP expressing bacteria was placed on an UV-table emitting light 302 nm (Figure 5). The picture shows CBD-sfGFP´s strong fluorescence at 302 nm UV-light.
Compatibility in Vibrio natriegens
In order to see if sfGFP worked in Vibrio natriegens using the strain Vmax, CBD-sfGFP (BBa_K3182108) and CBD-pCons-Aspink (BBa_K3182100) was ligated into the pUC19 vector and heat shocked into Vmax.Thereafter, the bacteria was spread onto LB-miller V2 agar dishes with 200 µg/ml carbenicillin and incubated in 37 °C for 16 hours. Both plates was put on an UV-table and illuminated in 302 nm (Figure 6). The picture below shows that the CBD-sfGFP bacteria, in comparison to the control CBD-pCons-AsPink, displays a strong green fluorescent color which verified that pUC19-CBD-sfGFP could successfully be heat shocked and expressed in Vmax.
Protein expression in different chassis
To measure the protein expression of T7-CBD-sfGFP in different bacteria and carbenicillin concentrations. BL21 (DE3) and Vibrio natriegens , using the strain Vmax, was grown in Falcon tubes to 0.5 OD600. Vmax was grown with two different carbenicillin concentrations, 200 and 600 µg/mL, while BL21 (DE3) had the same carbenicillin concentration of 100 µg/mL carbenicillin. The bacteria was induced with 1 mM IPTG and placed in a 96-well plate in 4 replicates with 200 µL per well. A spectrometry experiment was conducted and measured the fluorescence (excitation 470 nm,emission 550 nm) during 16 hours in 37 °C. The results seen below (Figure 7) shows that expression in Vmax with 600 µg/mL carbenicillin gave the highest protein yield. The most probable explanation for the increased protein yield for Vmax at 600 µg/mL carbenicillin is partially caused by the higher protein production of Vmax compared to BL21 (DE3). Another important factor was the use of an optimal concentration of carbenicillin (600 µg/mL) for Vmaxwhich retained the plasmid more efficiantly than Vmax at 200 µg/mL carbenicillin.
To measure the protein expression of T7-CBD-sfGFP in different bacteria and carbenicillin concentrations. BL21 (DE3) and Vibrio natriegens , using the strain Vmax, was grown in Falcon tubes to 0.5 OD600. Vmax was grown with two different carbenicillin concentrations, 200 and 600 µg/mL, while BL21 (DE3) had the same carbenicillin concentration of 100 µg/mL carbenicillin. The bacteria was induced with 1 mM IPTG and placed in a 96-well plate in 4 replicates with 200 µL per well. A spectrometry experiment was conducted and measured the fluorescence (excitation 470 nm,emission 550 nm) during 16 hours in 37 °C. The results seen below (Figure 7) shows that expression in Vmax with 600 µg/mL carbenicillin gave the highest protein yield. The most probable explanation for the increased protein yield for Vmax at 600 µg/mL carbenicillin is partially caused by the higher protein production of Vmax compared to BL21 (DE3). Another important factor was the use of an optimal concentration of carbenicillin (600 µg/mL) for Vmaxwhich retained the plasmid more efficiantly than Vmax at 200 µg/mL carbenicillin.
User Reviews
UNIQ50ba37f6fc4950cd-partinfo-00000005-QINU
UNIQ50ba37f6fc4950cd-partinfo-00000006-QINU