Difference between revisions of "Part:BBa K3147009"
| Line 5: | Line 5: | ||
| − | ===I. Part BBa_K3147009 | + | ===I. Part BBa_K3147009 function=== |
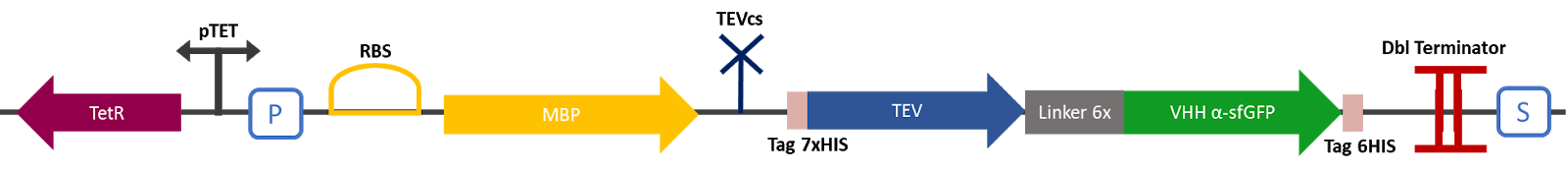
The 2019 Montpellier iGEM team made this construction in order to compare the basal activity of the TEV protease (BBa_ K1319004) with the same protease fused to a VHH nanobody. This construction produces an MBP fused to a TEV protease attached to a VHH specific for sfGFP. A TEV cutting site (BBa_ J18918) was added between the MBP and the protease. MBP increases the solubility of the fusion protein [1], preventing the aggregation of the protein of interest. This stabilizes the expression and the sequence of the produced MBP does not have a signal peptide, which allows the protein to remain in the cytosol. The TEV cutting site allows self-cleavage of MBP from TEV once the protein is produced [2]. A 7His affinity tag was added to C-terminus of the VHH to facilitate validation of protein production and MBP cleavage via Western blot and protein purification if desired. | The 2019 Montpellier iGEM team made this construction in order to compare the basal activity of the TEV protease (BBa_ K1319004) with the same protease fused to a VHH nanobody. This construction produces an MBP fused to a TEV protease attached to a VHH specific for sfGFP. A TEV cutting site (BBa_ J18918) was added between the MBP and the protease. MBP increases the solubility of the fusion protein [1], preventing the aggregation of the protein of interest. This stabilizes the expression and the sequence of the produced MBP does not have a signal peptide, which allows the protein to remain in the cytosol. The TEV cutting site allows self-cleavage of MBP from TEV once the protein is produced [2]. A 7His affinity tag was added to C-terminus of the VHH to facilitate validation of protein production and MBP cleavage via Western blot and protein purification if desired. | ||
| Line 22: | Line 22: | ||
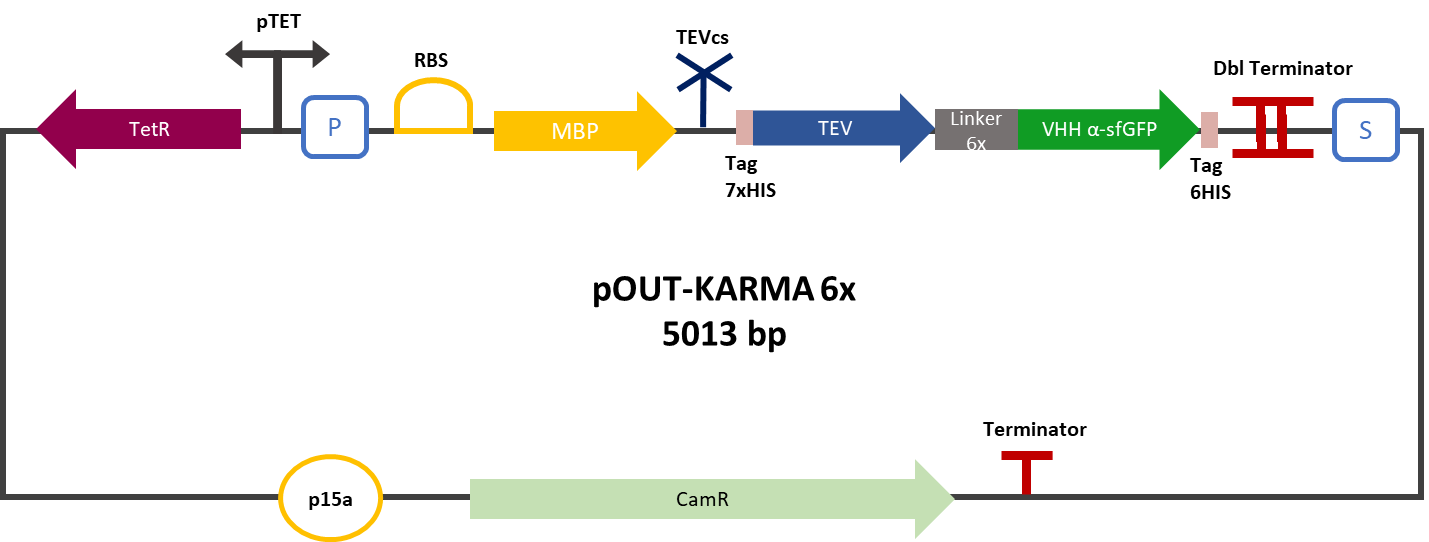
<div align="center"><b>Figure 2</b>: MBP-TEV-VHH reporter gene in its backbone pOUT18.</div> | <div align="center"><b>Figure 2</b>: MBP-TEV-VHH reporter gene in its backbone pOUT18.</div> | ||
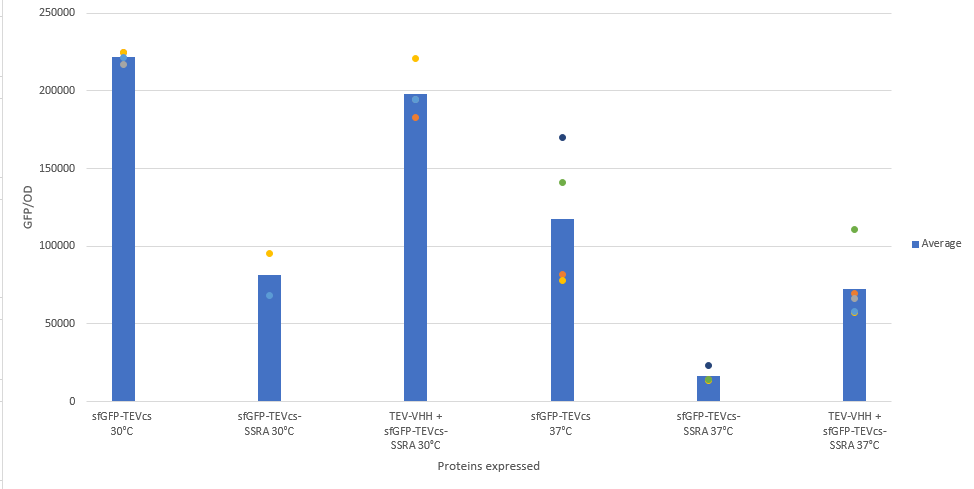
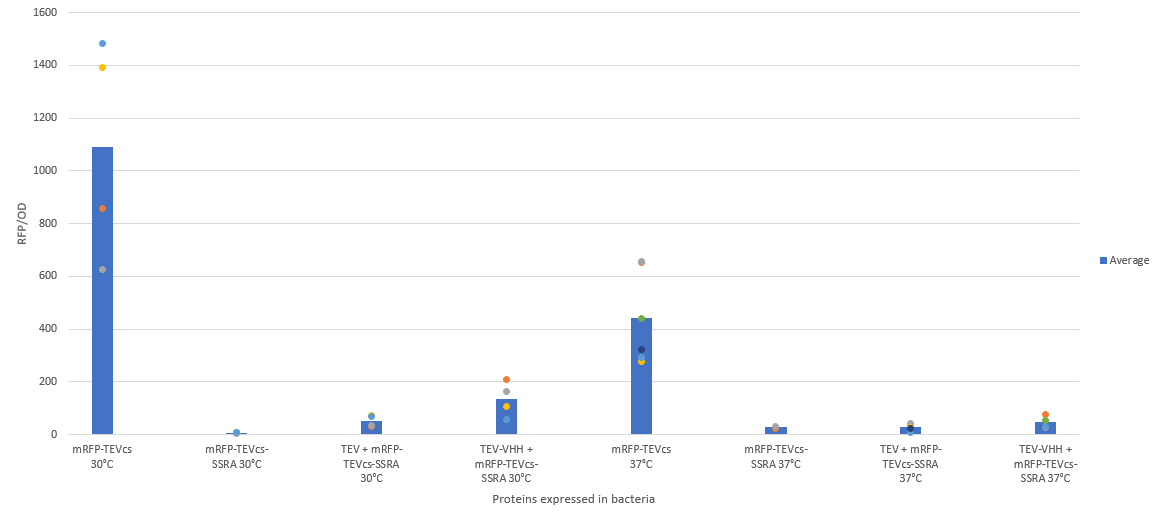
| − | The construction was cloned by Gibson Assembly in a pOUT18 vector under the control of a Tet-on promoter in order to control its expression. The experimental approach to test protease activity is to compare the fluorescence recovery of an | + | The construction was cloned by Gibson Assembly in a pOUT18 vector under the control of a Tet-on promoter in order to control its expression. The experimental approach to test protease activity is to compare the fluorescence recovery of an ssrA-tagged sfGFP by tag cleavage between MBP-TEV and MBP-TEV fused to a sfGFP targeting VHH. In this experiment, basal controls of maximum and minimum fluorescence of reporter genes were used. Fluorescence data are obtained by plate reader and are expressed in arbitrary units relative to optical density at 600 nm. The protease is expressed by inducing the Tet promoter with 50 ng/mL of aTc (anhydrotetracycline). The specificity of the VHH was also tested, for which we used the reporter genes mRFP1-TEVcs-SSRA (BBa_ K3147003) and mRFP1-TEVcs (BBa_ K3147004). The objective was to see if the activity of the TEV was improved by anchoring the TEV close to its cleavage site using the VHH. For this purpose we measured the fluorescence recovery between NEB10β strains of <i>E. coli</i> co-transformed with the reporter genes mentioned above. The protease is expressed by inducing the Tet promoter with 50 ng/mL of aTc (anhydrotetracycline). |
<div align="center">[[File:resultsfGFPK3147009.png|800px]]</div> | <div align="center">[[File:resultsfGFPK3147009.png|800px]]</div> | ||
Revision as of 14:46, 20 October 2019
I. Part BBa_K3147009 function
The 2019 Montpellier iGEM team made this construction in order to compare the basal activity of the TEV protease (BBa_ K1319004) with the same protease fused to a VHH nanobody. This construction produces an MBP fused to a TEV protease attached to a VHH specific for sfGFP. A TEV cutting site (BBa_ J18918) was added between the MBP and the protease. MBP increases the solubility of the fusion protein [1], preventing the aggregation of the protein of interest. This stabilizes the expression and the sequence of the produced MBP does not have a signal peptide, which allows the protein to remain in the cytosol. The TEV cutting site allows self-cleavage of MBP from TEV once the protein is produced [2]. A 7His affinity tag was added to C-terminus of the VHH to facilitate validation of protein production and MBP cleavage via Western blot and protein purification if desired.
II. Proof of function
The construction was cloned by Gibson Assembly in a backbone pOUT18 under the control of a TET ON promoter, in order to control its expression. The TEV protease used is optimized for expression in E. coli. The sequence contains cleavage mutation, S219V anti-self-cleavage. The TEV protease is a highly specific cysteine protease of the tabacco ech virus sequence. The protease is highly specific for the consensus sequence for its cleavage site which is ENLYFQ\G (BBa_ J18918) with \ indicating cleaved peptide binding. The VHH is a VHH directed against the sfGFP, it recognizes a conformational pattern.
The construction was cloned by Gibson Assembly in a pOUT18 vector under the control of a Tet-on promoter in order to control its expression. The experimental approach to test protease activity is to compare the fluorescence recovery of an ssrA-tagged sfGFP by tag cleavage between MBP-TEV and MBP-TEV fused to a sfGFP targeting VHH. In this experiment, basal controls of maximum and minimum fluorescence of reporter genes were used. Fluorescence data are obtained by plate reader and are expressed in arbitrary units relative to optical density at 600 nm. The protease is expressed by inducing the Tet promoter with 50 ng/mL of aTc (anhydrotetracycline). The specificity of the VHH was also tested, for which we used the reporter genes mRFP1-TEVcs-SSRA (BBa_ K3147003) and mRFP1-TEVcs (BBa_ K3147004). The objective was to see if the activity of the TEV was improved by anchoring the TEV close to its cleavage site using the VHH. For this purpose we measured the fluorescence recovery between NEB10β strains of E. coli co-transformed with the reporter genes mentioned above. The protease is expressed by inducing the Tet promoter with 50 ng/mL of aTc (anhydrotetracycline).
Reference
[1] Raran-Kurussi, Sreejith, et David S. Waugh. 2012. « The Ability to Enhance the Solubility of Its Fusion Partners Is an Intrinsic Property of Maltose-Binding Protein but Their Folding Is Either Spontaneous or Chaperone-Mediated » éd. Bostjan Kobe. PLoS ONE 7(11): e49589.
[2] Shih, Y.-P. 2005. « Self-Cleavage of Fusion Protein in Vivo Using TEV Protease to Yield Native Protein ». Protein Science 14(4): 936‑41.
[3] Yi, L. et al. 2013. « Engineering of TEV Protease Variants by Yeast ER Sequestration Screening (YESS) of Combinatorial Libraries ». Proceedings of the National Academy of Sciences 110(18): 7229‑34
[4] Kubala, M. H., Kovtun, O., Alexandrov, K., & Collins, B. M. (2010). Structural and thermodynamic analysis of the GFP:GFP-nanobody complex. Protein science : a publication of the Protein Society, 19(12), 2389–2401. doi:10.1002/pro.519
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 77
Illegal BsaI.rc site found at 2398
Illegal SapI.rc site found at 1541
Illegal SapI.rc site found at 1889