Difference between revisions of "Part:BBa K3196002"
GlacierHOLE (Talk | contribs) |
GlacierHOLE (Talk | contribs) |
||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3196002 short</partinfo> | <partinfo>BBa_K3196002 short</partinfo> | ||
| + | Small laccase gene | ||
<h1>'''Characterization'''</h1> | <h1>'''Characterization'''</h1> | ||
| + | Amplified from S. coelicolor. | ||
<h1>'''Usage and Biology'''</h1> | <h1>'''Usage and Biology'''</h1> | ||
Laccase is a multicopper oxidase, capable of catalyzing one-electron oxidation of a wide range of substrates to generate radicals while concomitantly reducing molecular oxygen to water<sup>[1]</sup>. Laccases are widely distributed in nature. They have been isolated from fungi, plants, insects and bacteria<sup>[2]</sup> of which fungal laccases are well characterized <sup>[3]</sup>. | Laccase is a multicopper oxidase, capable of catalyzing one-electron oxidation of a wide range of substrates to generate radicals while concomitantly reducing molecular oxygen to water<sup>[1]</sup>. Laccases are widely distributed in nature. They have been isolated from fungi, plants, insects and bacteria<sup>[2]</sup> of which fungal laccases are well characterized <sup>[3]</sup>. | ||
| Line 10: | Line 12: | ||
<h1>'''DNA Gel Electrophoretic '''</h1> | <h1>'''DNA Gel Electrophoretic '''</h1> | ||
| − | [[File:T--HUST-China--2019-DNA Gel Electrophoretic.png|400px|thumb| | + | [[File:T--HUST-China--2019-DNA Gel Electrophoretic.png|400px|thumb|center|Figure1:This is the result of the SacⅠ single endonuclease digestion plasmid of the TOP10 strain, we will extract the gel and obtain the aimed DNA. ]] |
| − | [[File:T--HUST-China--2019-pichiaDNA Gel Electrophoretic.png|400px|thumb|center|The image above shows the successful transformation of the target gene into the Pichia pastoris genome, including SLAC FLO10, SLAC PHO5 | + | [[File:T--HUST-China--2019-pichiaDNA Gel Electrophoretic.png|400px|thumb|center|Figure2:The image above shows the successful transformation of the target gene into the Pichia pastoris genome, including SLAC FLO10, SLAC PHO5 apro, and SLAC. ]] |
<h1>'''SDS-PAGE'''</h1> | <h1>'''SDS-PAGE'''</h1> | ||
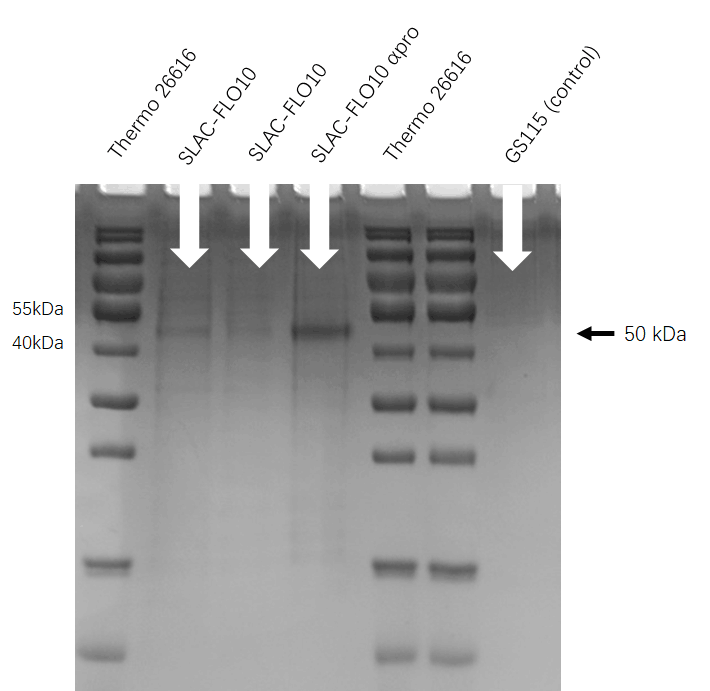
| − | [[File:T--HUST-China--2019-SLAC-SDS-PAGE.jpg |400px|thumb|center| | + | [[File:T--HUST-China--2019-SLAC-SDS-PAGE.jpg |400px|thumb|center|Figure3:the SDS-page result shows that Pichia pastoris have successfully secrete SLAC protein into superfluous liquid.]] |
<h1>'''Enzyme Activity'''</h1> | <h1>'''Enzyme Activity'''</h1> | ||
| − | [[File|400px|thumb|center| | + | [[File:T--HUST-China--2019-SLAC enzyme activity.png|400px|thumb|center|Figure4:these four pictures shows the enzyme activity changes with the time. The four kinds of Pichia pastoris have the different curve, and the most activity strain is FLO10-apro, PHO5apro shows a more delayed increase on enzyme activity.]] |
| + | |||
Revision as of 04:38, 20 October 2019
SLAC
Small laccase gene
Characterization
Amplified from S. coelicolor.
Usage and Biology
Laccase is a multicopper oxidase, capable of catalyzing one-electron oxidation of a wide range of substrates to generate radicals while concomitantly reducing molecular oxygen to water[1]. Laccases are widely distributed in nature. They have been isolated from fungi, plants, insects and bacteria[2] of which fungal laccases are well characterized [3]. Bacterial laccases, such as the small laccase from S. coelicolor (SLAC), can be useful alternatives to fungal laccases. SLAC exhibits some desirable characteristics such as activity at high temperatures and alkaline pH [4], making it a potential candidate in biotechnological applications. Extracellular expression could be a desirable alternative for expression of foreign proteins since it simplifies purification steps [5]. P. pastoris is usually the preferred host for the production of industrial enzymes.
DNA Gel Electrophoretic
SDS-PAGE
Enzyme Activity
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 345