Difference between revisions of "Part:BBa K3183200"
(→Characterisation) |
(→Characterisation) |
||
| Line 31: | Line 31: | ||
While <i>L. reuteri</i> grows to a smaller maximum OD600 than <i>B. subtilis</i>, there is no discernable change in OD600 due to endolysin . Notably, the effect of lysozyme on <i>L. reuteri</i> is relatively small due to its thick bacterial cell wall, and we were unable to show a killing effect. For this reason, ampicillin was chosen as our positive control.]]<br><br> | While <i>L. reuteri</i> grows to a smaller maximum OD600 than <i>B. subtilis</i>, there is no discernable change in OD600 due to endolysin . Notably, the effect of lysozyme on <i>L. reuteri</i> is relatively small due to its thick bacterial cell wall, and we were unable to show a killing effect. For this reason, ampicillin was chosen as our positive control.]]<br><br> | ||
| − | + | ||
| + | |||
| + | |||
| + | Finally, we were fortunate enough to obtain scanning electron microscopy (SEM) images thanks to Errin Johnson at the Dunn School of Pathology. After performing our endolysin killing assay, we fixed the cells onto glass slides and imaged them. We chose this as a qualitative method to determine if CD27L had a similar effect on endolysin killing as lysozyme<sup>4</sup>. Below shows images of <i>B. subtilis</i> following CD27L endolysin treatment (Fig. 8). Here, we demonstrated some changes in <i>B. subtilis</i> cell morphology, but further investigation is needed to explore these effects.<br><br><br> | ||
[[File:BBa_K3183200_Lreuteri.png|thumb|right|700px|'''Figure 8:''' CD27L Endolysin Killing Assay on <i>Bacillus subtilis</i> versus <i>Lactobacillus reuteri</i>; | [[File:BBa_K3183200_Lreuteri.png|thumb|right|700px|'''Figure 8:''' CD27L Endolysin Killing Assay on <i>Bacillus subtilis</i> versus <i>Lactobacillus reuteri</i>; | ||
While <i>L. reuteri</i> grows to a smaller maximum OD600 than <i>B. subtilis</i>, there is no discernable change in OD600 due to endolysin . Notably, the effect of lysozyme on <i>L. reuteri</i> is relatively small due to its thick bacterial cell wall, and we were unable to show a killing effect. For this reason, ampicillin was chosen as our positive control.]]<br> | While <i>L. reuteri</i> grows to a smaller maximum OD600 than <i>B. subtilis</i>, there is no discernable change in OD600 due to endolysin . Notably, the effect of lysozyme on <i>L. reuteri</i> is relatively small due to its thick bacterial cell wall, and we were unable to show a killing effect. For this reason, ampicillin was chosen as our positive control.]]<br> | ||
Revision as of 22:27, 19 October 2019
CD27L with N-Terminal 6-His Tag and SpyTag
CD27L with N-Terminal 6-His Tag and SpyTag
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterisation
Expression of CD27L was shown to
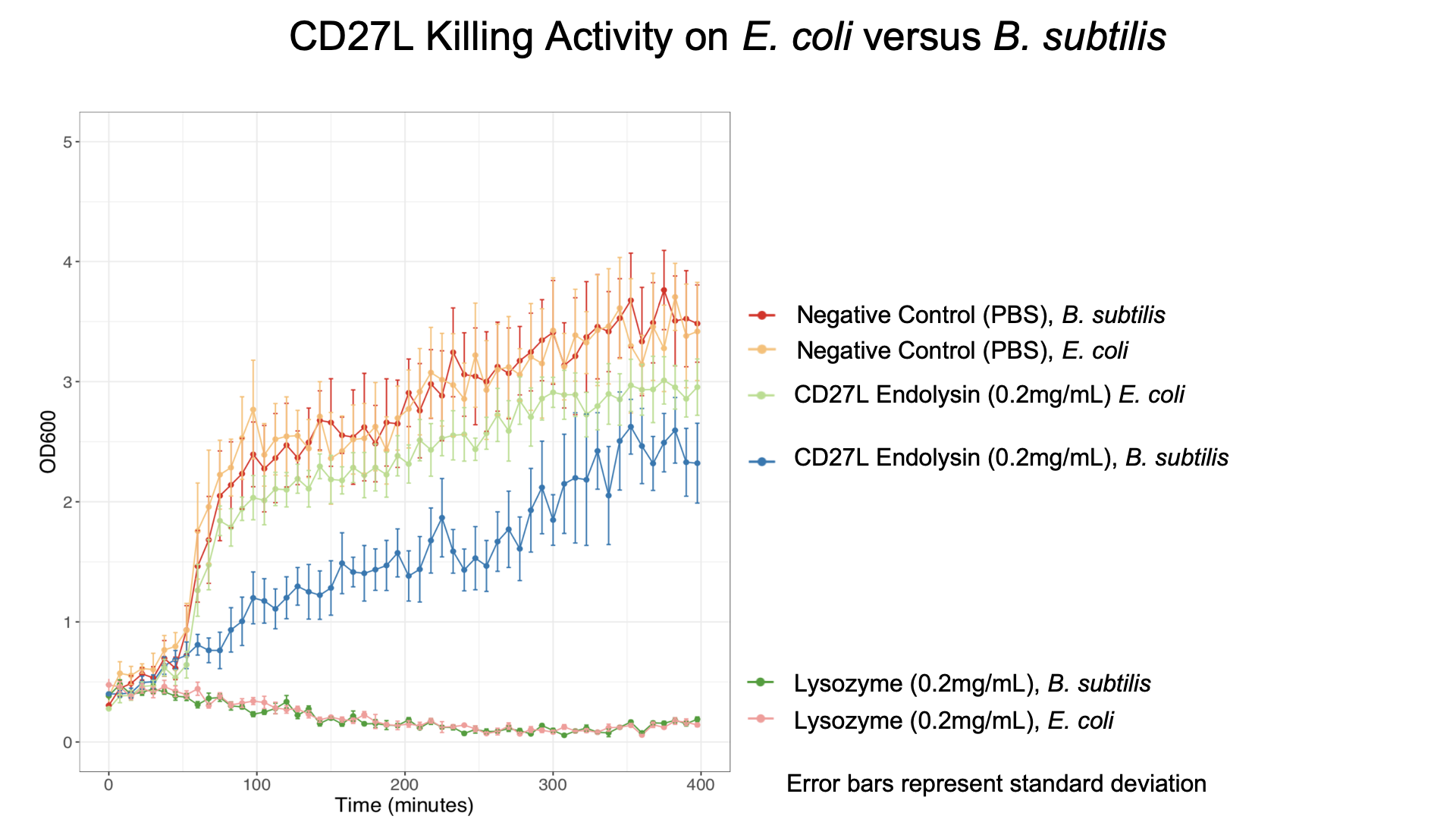
To get more information about the killing kinetics of the CD27L endolysin, we titrated our killing assay with increasing concentrations of CD27L endolysin. This titration showed measurable effect on B. subtilis cultures in LB broth with final endolysin concentrations greater than 0.2mg/mL (Fig. 5). Rate constants regarding how much the endolysin inhibited growth were subsequently used to inform our model (Table 2).
From this data, we were able to obtain kinetic constants for endolysin killing, which were subsequently used in our mathematical models.

We also showed that endolysin was specific to B. subtilis using a killing assay involving E. coli, B. subtilis, and our chassis Lactobacillus reuteri (Fig. 6 & 7).

Finally, we were fortunate enough to obtain scanning electron microscopy (SEM) images thanks to Errin Johnson at the Dunn School of Pathology. After performing our endolysin killing assay, we fixed the cells onto glass slides and imaged them. We chose this as a qualitative method to determine if CD27L had a similar effect on endolysin killing as lysozyme4. Below shows images of B. subtilis following CD27L endolysin treatment (Fig. 8). Here, we demonstrated some changes in B. subtilis cell morphology, but further investigation is needed to explore these effects.

Part Improvement of BBa_K895004
A fundamental crux of the ProQuorum system is the expression of the endolysin which is responsible for the actual lysis and consequent killing of the pathogenic C. difficile. Thus, there is a clear need for an endolysin that fulfills the criteria of being:
1. optimally expressed
2. optimally secreted and
3. optimally effective against C.difficile
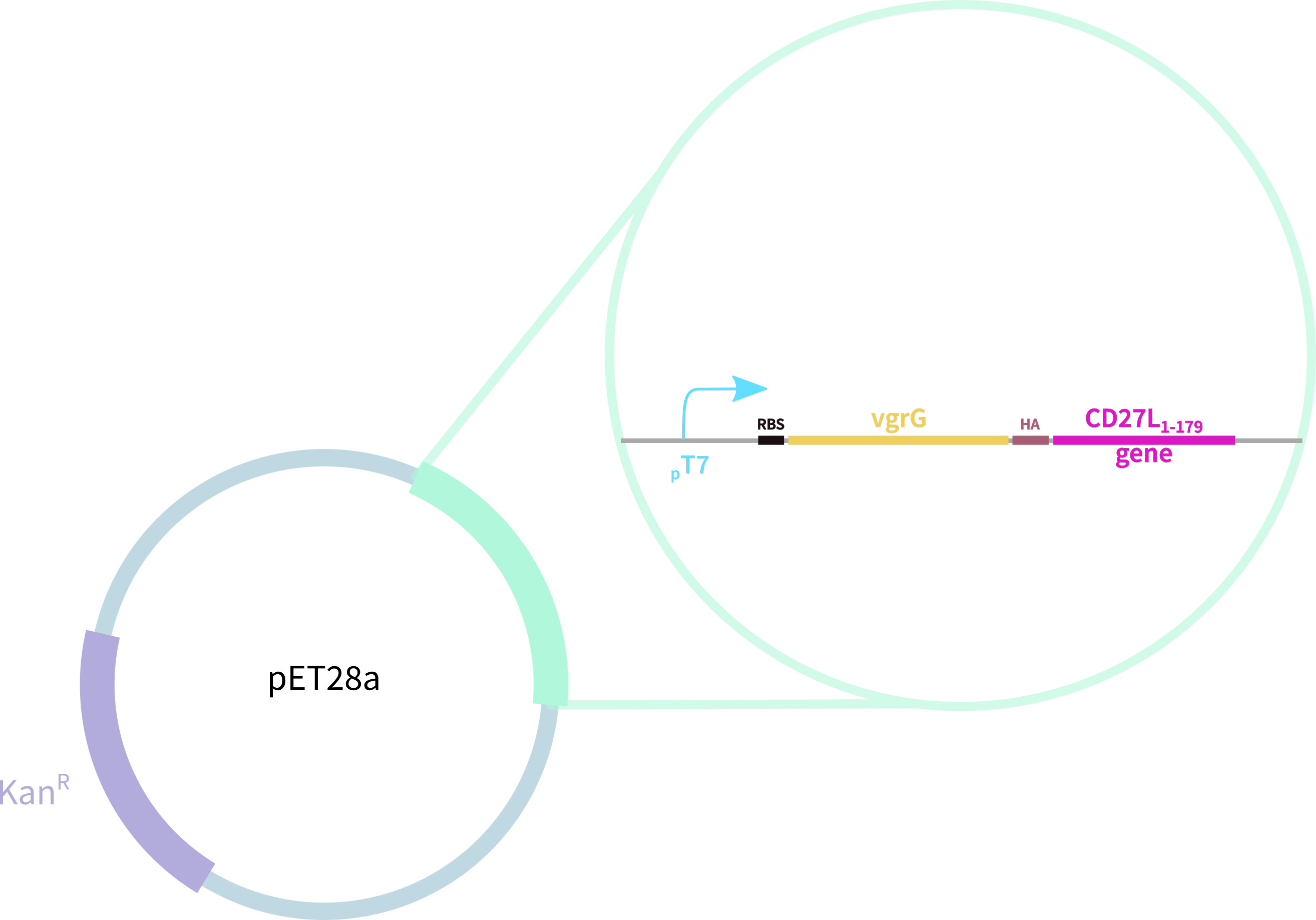
Thus, we redesigned the BBa_K895005 part submitted by the 2012 Dundee iGEM team, which consists of:
1. truncated form of the CD27L endolysin, shown to increase both efficacy and host range2
2. Type VI secretion protein derived from S. typhimurium
3. Hemagglutinin (HA) Tag for Western Blotting
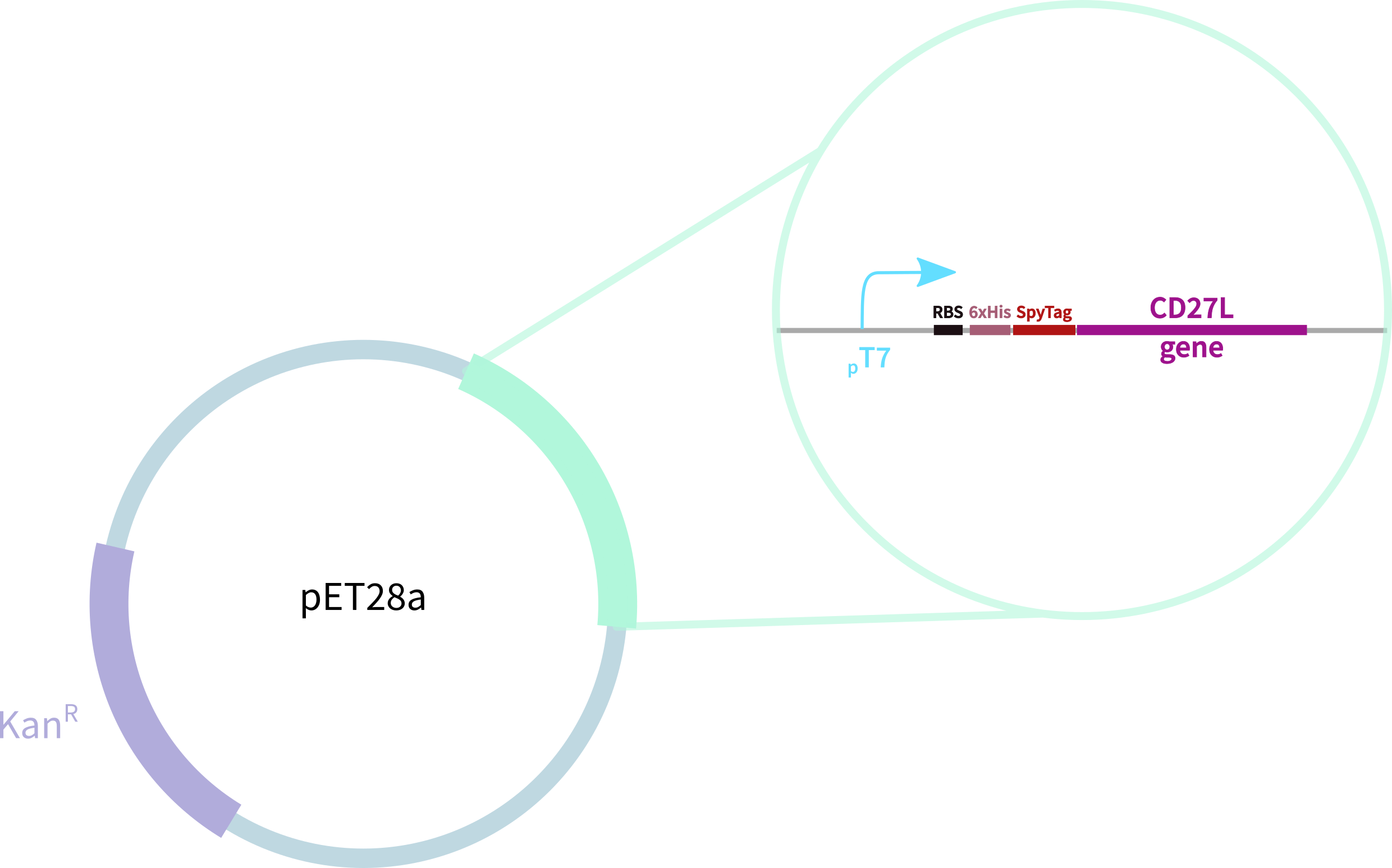
In contrast, our novel part BBa_K3183009 (CD27L) encodes:
1. full-length form of the CD27L endolysin
2. SpyTag for purification, oligomerisation, and other SpyCatcher applications
3. 6-His tag for easy purification
Incorporating both of these parts into pET28A, our expression vector, we planned on testing the relative efficacies of B.subtilis killing as per our killing assays. Thus, we followed our generic pipeline of:
1. transformation of both constructs into E.coli (BL21 (DE3)-RIPL Competent E.coli)
2. miniprep + sequencing to verify successful transformation
3. induction of expression with IPTG
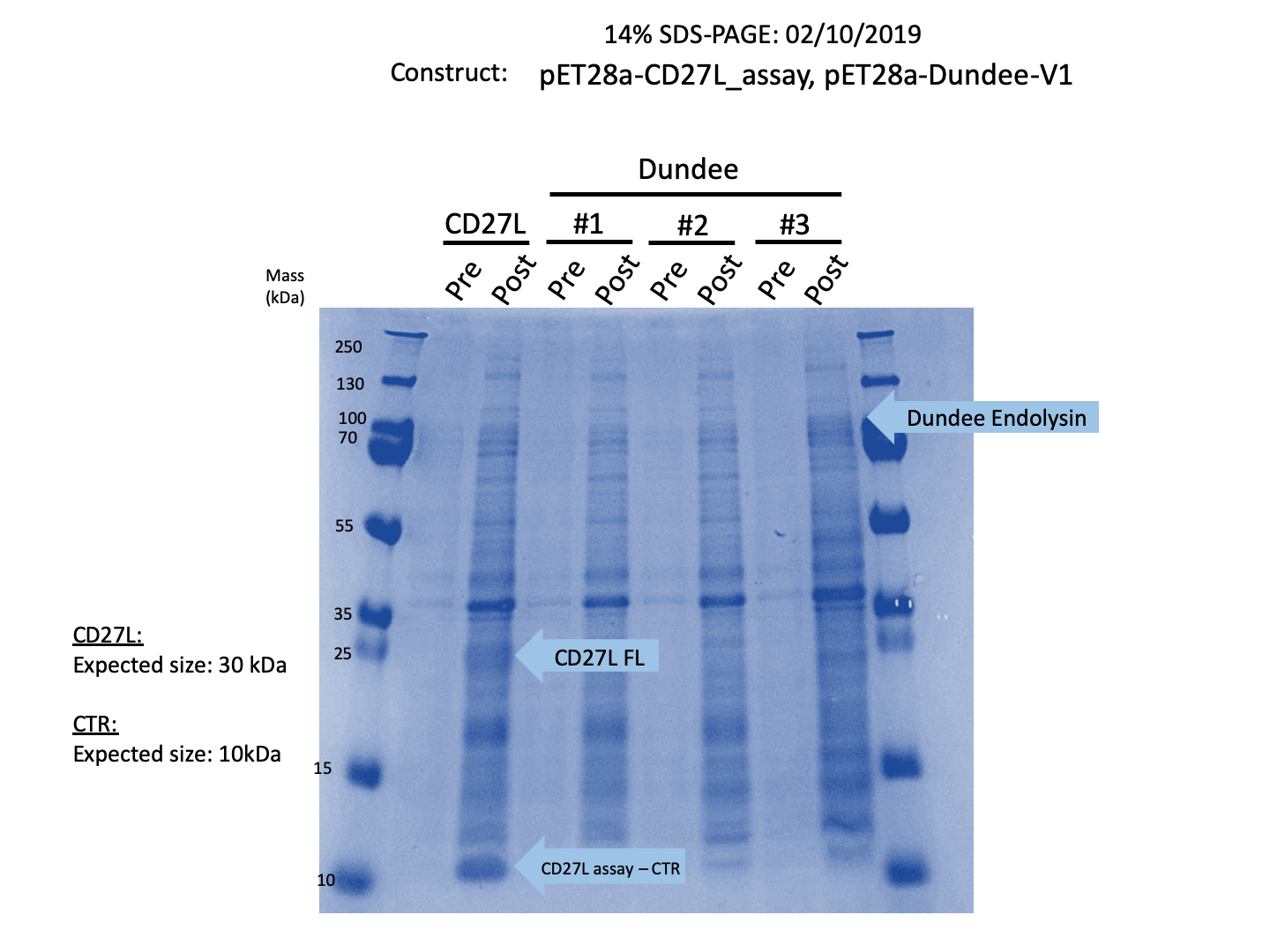
Thus, we sought out the endolysin in an existing part made by the 2012 iGEM Team Dundee. However, despite identical growth and induction conditions, expression of the Dundee 2012 iGEM endolysin was unsuccessful, even in triplicate, as shown in Figure 1. In contrast, expression of the CD27L_Assay part was successful and quantitatively verified via both mass spectrometry and BCA assay, as in the Results page.
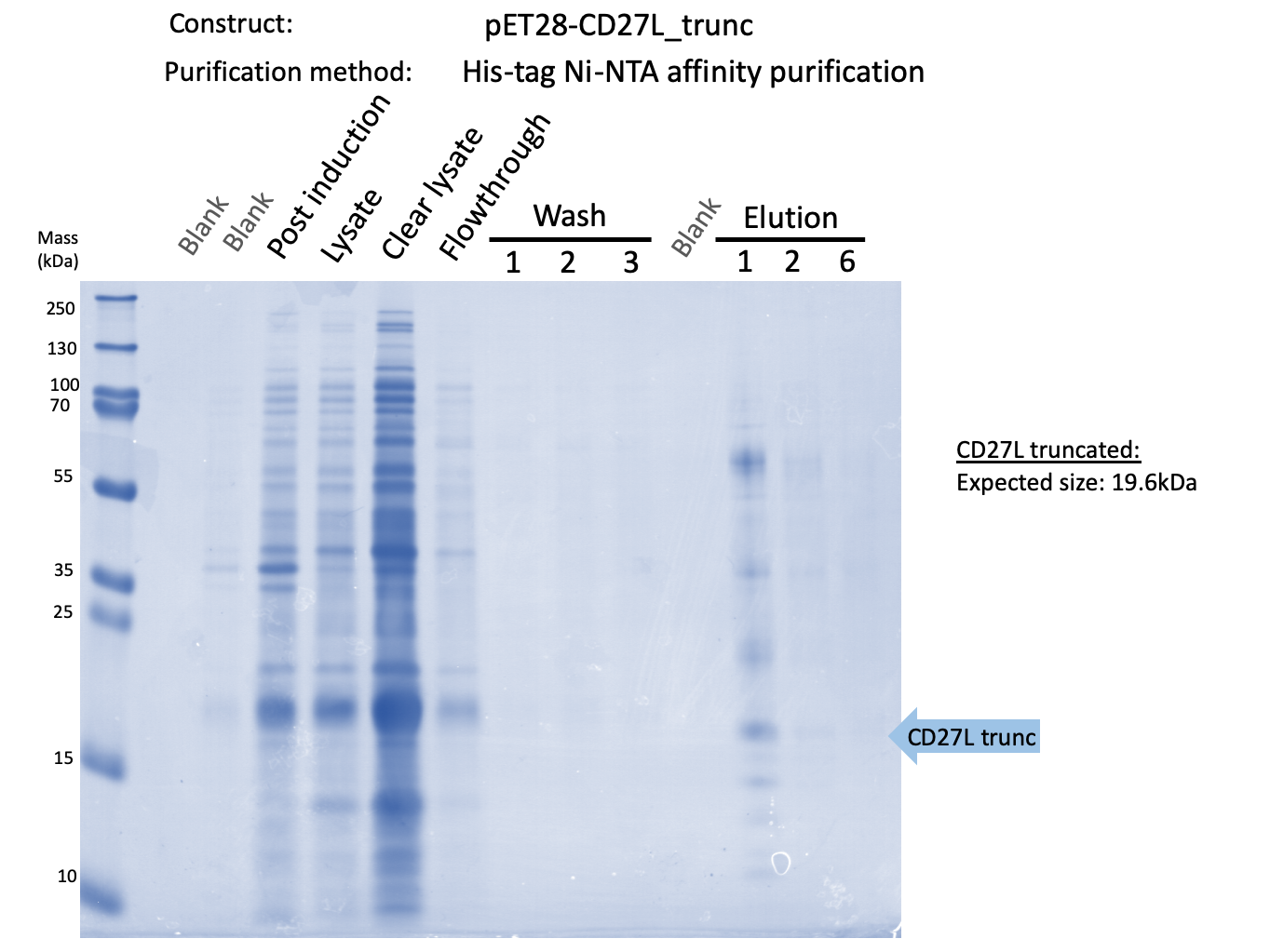
Thus, to solve this issue, we decided to attempt expression of only the truncated endolysin from the Dundee 2012 Biobrick, without its substantially large Type VI secretion tag. This resulted in BBa_K31830012 (CD27L1-179</sib<). Upon analysis, there is evident expression of the truncated endolysin as seen in Figure 2. However, given identical conditions of growth and induction, there appears to be greater expression of the full-length endolysin, which may perhaps indicate greater stability.
As a further improvement, we decided to measure the killing efficacy of the CD27L_Assay endolysin on B. subtilis (our surrogate target) as no killing data was provided for the Dundee 2012 BBa_K895005 part. As seen in Figure 3, there is a substantial decrease in OD600 over time relative to the negative controls.
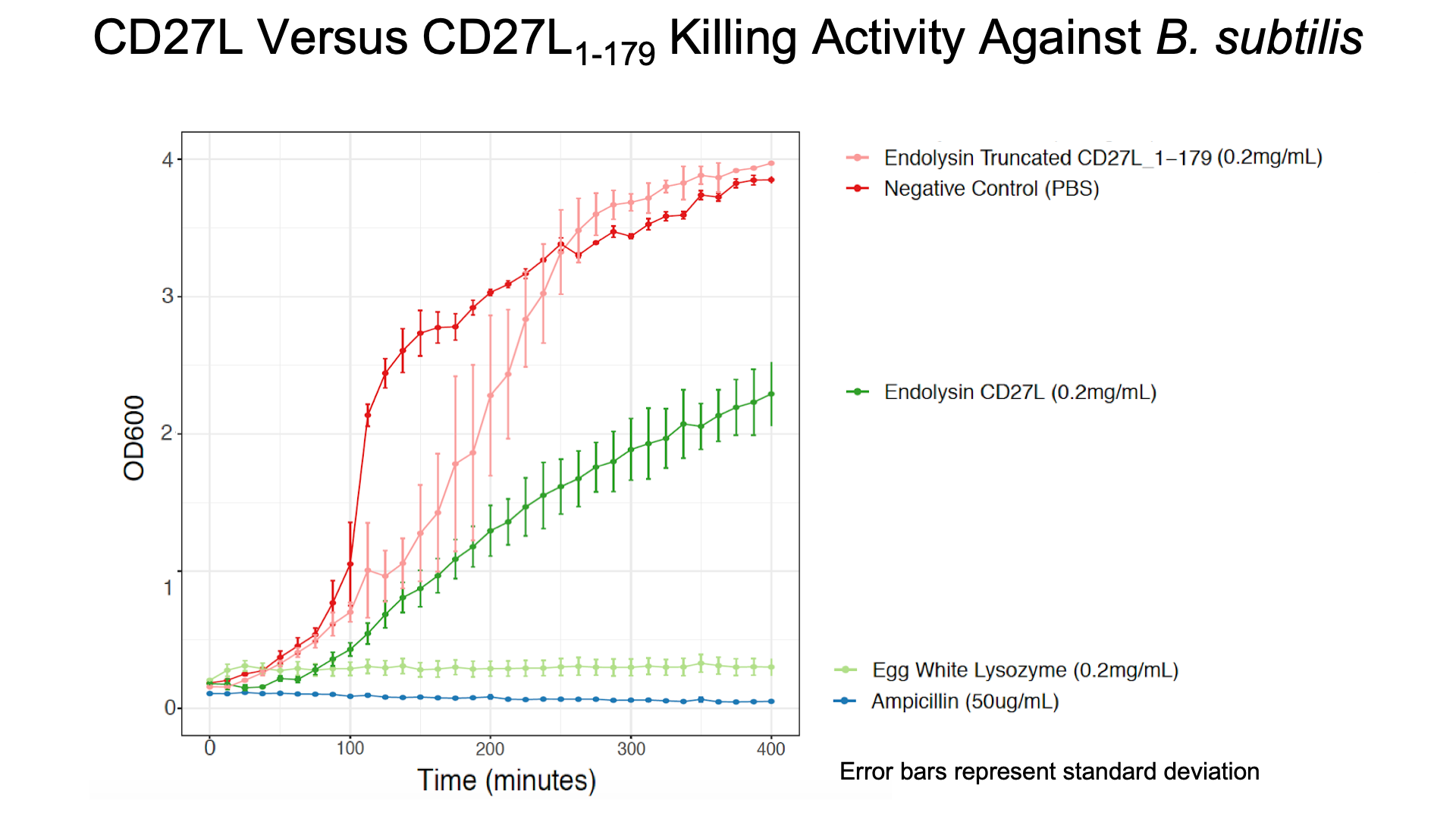
The truncated CD27L1-179 shows decreased growth relative to the negative control; however, log-phase growth resumes after 150 minutes. CD27L endolysin results in decreased growth during the first 100 minutes, and growth thereafter appears to be linear. This may point to inhibited log-phase growth in subsequent generations of B. subtilis following CD27L exposure.