Difference between revisions of "Part:BBa K2959005"
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2959005 short</partinfo> | <partinfo>BBa_K2959005 short</partinfo> | ||
<p align="justify"> | <p align="justify"> | ||
This composite part consists of a lacI regulated promoter, ribosome binding site, a coding sequence for <i>Pinus sylvestris</i> Defensin 1 as a fusion protein with a 6x His-Tag, a second ribosome binding site, a coding sequence for Erv1p, and a double terminator. This construct allows the expression of PsDef1, an antifungal peptide from Scots pine seeds, in <i>E. coli</i>. Expression can be positively regulated by the addition of IPTG or lactose thanks to the lacl regulated promoter. The fusion of PsDef1 to a 6x His-Tag is intended for the purification of the peptide by immobilized metal affinity chromatography. | This composite part consists of a lacI regulated promoter, ribosome binding site, a coding sequence for <i>Pinus sylvestris</i> Defensin 1 as a fusion protein with a 6x His-Tag, a second ribosome binding site, a coding sequence for Erv1p, and a double terminator. This construct allows the expression of PsDef1, an antifungal peptide from Scots pine seeds, in <i>E. coli</i>. Expression can be positively regulated by the addition of IPTG or lactose thanks to the lacl regulated promoter. The fusion of PsDef1 to a 6x His-Tag is intended for the purification of the peptide by immobilized metal affinity chromatography. | ||
| − | + | <br> | |
Since PsDef1 is a peptide with four disulfide bonds<sup>1</sup>, a modification hard to replicate on prokaryotic expression systems, the construct allows for the co-expression of the peptide with a truncated version of Erv1p. This is a protein from Saccharomyces cerevisiae capable of catalyzing the formation of disulfide bonds<sup>2</sup>. | Since PsDef1 is a peptide with four disulfide bonds<sup>1</sup>, a modification hard to replicate on prokaryotic expression systems, the construct allows for the co-expression of the peptide with a truncated version of Erv1p. This is a protein from Saccharomyces cerevisiae capable of catalyzing the formation of disulfide bonds<sup>2</sup>. | ||
| − | + | </p> | |
| + | <br> | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | <p align="justify"> | ||
PsDef1 is present in <i>Pinus sylvestris</i> seeds, vegetative and generative organs. The defensin encodes a protein of 83 amino acids in length, whose first 33 amino acids correspond to the ‘N-terminal signal peptide, it also has eight highly conserved cysteines residues that are predicted to form four disulfide bonds C3–C49, C14–C34, C20–C43, and C24–C45<sup>1</sup>, which stabilize its structure making it resistant to environmental changes<sup>2</sup>. Its conformation consists in a cysteine-stabilized αβ-motif (CSαβ) with a prominent α-helix and a triple-stranded antiparallel β-sheet that is stabilized by the 4 disulfide bonds<sup>1</sup>.</p> | PsDef1 is present in <i>Pinus sylvestris</i> seeds, vegetative and generative organs. The defensin encodes a protein of 83 amino acids in length, whose first 33 amino acids correspond to the ‘N-terminal signal peptide, it also has eight highly conserved cysteines residues that are predicted to form four disulfide bonds C3–C49, C14–C34, C20–C43, and C24–C45<sup>1</sup>, which stabilize its structure making it resistant to environmental changes<sup>2</sup>. Its conformation consists in a cysteine-stabilized αβ-motif (CSαβ) with a prominent α-helix and a triple-stranded antiparallel β-sheet that is stabilized by the 4 disulfide bonds<sup>1</sup>.</p> | ||
| Line 18: | Line 19: | ||
<p align="justify"> | <p align="justify"> | ||
The ERV1 gene from <i>Saccharomyces cerevisiae</i> encodes for a 189 amino acids protein, Erv1p, that is involved in different processes of mitochondrial biogenesis and maintenance<sup>2, 6</sup>. The protein shows a flavin-linked sulfhydryl oxidase enzymatic activity linked to the carboxy-terminal domain. Thus, Erv1p is capable of oxidizing thiol groups in proteins and catalyzing disulfide bond formation. A 15 kDa truncated version of Erv1p, consisting of the 117 amino acid residues carboxy-terminal domain, shows a similar or improved sulfhydryl oxidase activity compared to the full length protein<sup>2</sup>.</p> | The ERV1 gene from <i>Saccharomyces cerevisiae</i> encodes for a 189 amino acids protein, Erv1p, that is involved in different processes of mitochondrial biogenesis and maintenance<sup>2, 6</sup>. The protein shows a flavin-linked sulfhydryl oxidase enzymatic activity linked to the carboxy-terminal domain. Thus, Erv1p is capable of oxidizing thiol groups in proteins and catalyzing disulfide bond formation. A 15 kDa truncated version of Erv1p, consisting of the 117 amino acid residues carboxy-terminal domain, shows a similar or improved sulfhydryl oxidase activity compared to the full length protein<sup>2</sup>.</p> | ||
| − | + | <br> | |
| − | == | + | ==<b>Characterization of Expressible Pinus sylvestris Defensin 1 with Erv1p</b>== |
<p align= "justify"> | <p align= "justify"> | ||
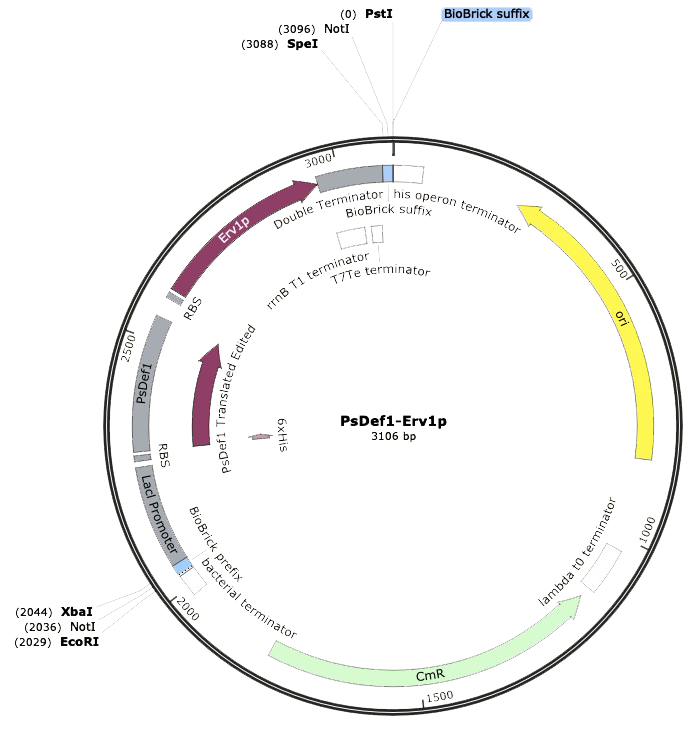
Our DNA sequence PsDef1-Erv1p was synthesized by IDT®️ with the Biobrick prefix and suffix flanking the composite part. This made possible the correct digestion with restriction enzymes EcoRI-HF and PstI. After the digestion, ligation was performed with T7 ligase in order to place our construct into the pSB1C3 linearized backbone with chloramphenicol resistance, which was previously digested with the same restriction enzymes. Using the SnapGene®️ software, we could model our ligated expression plasmid, and the final part resulted in a sequence of 3,106 bp. Thereupon, <i>Escherichia coli</i> SHuffle was transformed by heat shock for following antibiotic selection of clones. | Our DNA sequence PsDef1-Erv1p was synthesized by IDT®️ with the Biobrick prefix and suffix flanking the composite part. This made possible the correct digestion with restriction enzymes EcoRI-HF and PstI. After the digestion, ligation was performed with T7 ligase in order to place our construct into the pSB1C3 linearized backbone with chloramphenicol resistance, which was previously digested with the same restriction enzymes. Using the SnapGene®️ software, we could model our ligated expression plasmid, and the final part resulted in a sequence of 3,106 bp. Thereupon, <i>Escherichia coli</i> SHuffle was transformed by heat shock for following antibiotic selection of clones. | ||
| Line 26: | Line 27: | ||
<center><b>Figure 1.</b>SnapGene®️ map of BioBrick BBa_K2959005</center> | <center><b>Figure 1.</b>SnapGene®️ map of BioBrick BBa_K2959005</center> | ||
| − | |||
| − | |||
| − | |||
| + | <p align="justify"> | ||
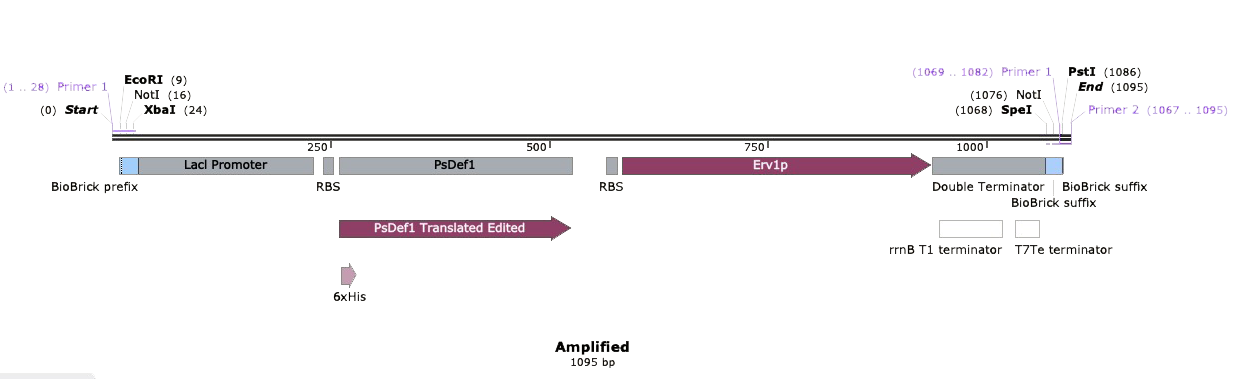
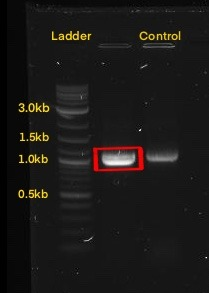
| + | The next step was to amplify our BioBrick sequence through colony PCR performed upon our transformed cells to confirm the presence of our expression plasmid inside of our chassis. With the help of the specific forward Biobrick prefix [BBa_G1004] and the specific reverse Biobrick suffix [BBa_G1005], we were able to amplify our sequence exclusively. Through an agarose gel we confirmed the correct transformation. The PCR action from SnapGene®️ was used to predict the size of the amplified sequences which resulted in a size of 1,095 for PsDef1+Erv1p.</p> | ||
<center>[[File:T--Tec-Chihuahua--psdef1.pcr..png|600px]] | <center>[[File:T--Tec-Chihuahua--psdef1.pcr..png|600px]] | ||
[[File:T--Tec-Chihuahua--ELECTROFORESISPsDefE.jpeg|200px]]</center> | [[File:T--Tec-Chihuahua--ELECTROFORESISPsDefE.jpeg|200px]]</center> | ||
<center><b>Figure 2.</b>(On the left) SnapGene®️ amplification through PCR of BBa_K2959005. (On the right) Agarose gel electrophoresis of BBa_K2959005 compared with NEB Quick-Load Purple 1Kb Plus DNA Ladder, where the highlighted band corresponds to approximately 1,095 bp.</center> | <center><b>Figure 2.</b>(On the left) SnapGene®️ amplification through PCR of BBa_K2959005. (On the right) Agarose gel electrophoresis of BBa_K2959005 compared with NEB Quick-Load Purple 1Kb Plus DNA Ladder, where the highlighted band corresponds to approximately 1,095 bp.</center> | ||
| − | + | <br> | |
| + | ===Protein production=== | ||
| + | <b>IPTG Induction and Extraction</b> | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | jfjdfdjskjdshfjdhfjdjkdfbdbhdfhdgfhdbfdhfdg | ||
| + | </p> | ||
<!-- --> | <!-- --> | ||
| Line 40: | Line 46: | ||
===Sequence and Features=== | ===Sequence and Features=== | ||
<partinfo>BBa_K2959005 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2959005 SequenceAndFeatures</partinfo> | ||
| − | + | <br> | |
| + | <b> | ||
===References=== | ===References=== | ||
| − | + | </b> | |
1. Khairutdinov, B. I., Ermakova, E. A., Yusypovych, Y. M., Bessolicina, E. K., Tarasova, N. B., Toporkova, Y. Y., ... & Nesmelova, I. V. (2017). NMR structure, conformational dynamics, and biological activity of PsDef1 defensin from Pinus sylvestris. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1865(8), 1085-1094. doi:10.1016/j.bbapap.2017.05.012 | 1. Khairutdinov, B. I., Ermakova, E. A., Yusypovych, Y. M., Bessolicina, E. K., Tarasova, N. B., Toporkova, Y. Y., ... & Nesmelova, I. V. (2017). NMR structure, conformational dynamics, and biological activity of PsDef1 defensin from Pinus sylvestris. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1865(8), 1085-1094. doi:10.1016/j.bbapap.2017.05.012 | ||
<br> | <br> | ||
| Line 55: | Line 62: | ||
6. Lisowsky, T. (1996). Removal of an intron with unique 3′ branch site creates an amino‐terminal protein sequence directing the scERV1 gene product to mitochondria. Yeast, 12(15), 1501-1510. doi: 10.1002/(SICI)1097-0061(199612)12:15%3C1501::AID-YEA40%3E3.0.CO;2-H</p> | 6. Lisowsky, T. (1996). Removal of an intron with unique 3′ branch site creates an amino‐terminal protein sequence directing the scERV1 gene product to mitochondria. Yeast, 12(15), 1501-1510. doi: 10.1002/(SICI)1097-0061(199612)12:15%3C1501::AID-YEA40%3E3.0.CO;2-H</p> | ||
<br> | <br> | ||
| − | |||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
<partinfo>BBa_K2959005 parameters</partinfo> | <partinfo>BBa_K2959005 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
Revision as of 21:23, 19 October 2019
Expressible Pinus sylvestris Defensin 1 with Erv1p
This composite part consists of a lacI regulated promoter, ribosome binding site, a coding sequence for Pinus sylvestris Defensin 1 as a fusion protein with a 6x His-Tag, a second ribosome binding site, a coding sequence for Erv1p, and a double terminator. This construct allows the expression of PsDef1, an antifungal peptide from Scots pine seeds, in E. coli. Expression can be positively regulated by the addition of IPTG or lactose thanks to the lacl regulated promoter. The fusion of PsDef1 to a 6x His-Tag is intended for the purification of the peptide by immobilized metal affinity chromatography.
Since PsDef1 is a peptide with four disulfide bonds1, a modification hard to replicate on prokaryotic expression systems, the construct allows for the co-expression of the peptide with a truncated version of Erv1p. This is a protein from Saccharomyces cerevisiae capable of catalyzing the formation of disulfide bonds2.
Usage and Biology
PsDef1 is present in Pinus sylvestris seeds, vegetative and generative organs. The defensin encodes a protein of 83 amino acids in length, whose first 33 amino acids correspond to the ‘N-terminal signal peptide, it also has eight highly conserved cysteines residues that are predicted to form four disulfide bonds C3–C49, C14–C34, C20–C43, and C24–C451, which stabilize its structure making it resistant to environmental changes2. Its conformation consists in a cysteine-stabilized αβ-motif (CSαβ) with a prominent α-helix and a triple-stranded antiparallel β-sheet that is stabilized by the 4 disulfide bonds1.
Endogenous PsDef1 causes morphological changes in fungi mycelium when interacting with the sphingolipid membrane on fungal cell, disrupting the membrane integrity. Antifungal activity towards phytopathogenic fungi Botrytis cinerea, Fusarium oxysporum, Fusarium solani, and Heterobasidion annosum has been demonstrated; as well as its antimicrobial activity against Gram-positive (Bacillus pumilus) and Gram-negative bacteria (Pectobacterium carotovorum, Pseudomonas fluorescens)1.
For a successful expression in E. coli, it is indispensable to avoid interactions that result in the aggregation of folding intermediates. Disulfide bonds can be involved in the structural, catalytic and signaling roles of the protein, but the formation of disulfide bonds can have certain problems that can cause misfolding, aggregation and low yields during the production of the protein4. The usage of Erv1p has been proposed as a mechanism for the formation of disulfide bonds in recombinant proteins in prokaryotic expression systems. It has been shown that, thanks to its ability to form disulfide bonds de novo, co-expression of Erv1p allows the proper formation of disulfide bonded proteins in the cytoplasm of E. coli5.
The ERV1 gene from Saccharomyces cerevisiae encodes for a 189 amino acids protein, Erv1p, that is involved in different processes of mitochondrial biogenesis and maintenance2, 6. The protein shows a flavin-linked sulfhydryl oxidase enzymatic activity linked to the carboxy-terminal domain. Thus, Erv1p is capable of oxidizing thiol groups in proteins and catalyzing disulfide bond formation. A 15 kDa truncated version of Erv1p, consisting of the 117 amino acid residues carboxy-terminal domain, shows a similar or improved sulfhydryl oxidase activity compared to the full length protein2.
Characterization of Expressible Pinus sylvestris Defensin 1 with Erv1p
Our DNA sequence PsDef1-Erv1p was synthesized by IDT®️ with the Biobrick prefix and suffix flanking the composite part. This made possible the correct digestion with restriction enzymes EcoRI-HF and PstI. After the digestion, ligation was performed with T7 ligase in order to place our construct into the pSB1C3 linearized backbone with chloramphenicol resistance, which was previously digested with the same restriction enzymes. Using the SnapGene®️ software, we could model our ligated expression plasmid, and the final part resulted in a sequence of 3,106 bp. Thereupon, Escherichia coli SHuffle was transformed by heat shock for following antibiotic selection of clones.
The next step was to amplify our BioBrick sequence through colony PCR performed upon our transformed cells to confirm the presence of our expression plasmid inside of our chassis. With the help of the specific forward Biobrick prefix [BBa_G1004] and the specific reverse Biobrick suffix [BBa_G1005], we were able to amplify our sequence exclusively. Through an agarose gel we confirmed the correct transformation. The PCR action from SnapGene®️ was used to predict the size of the amplified sequences which resulted in a size of 1,095 for PsDef1+Erv1p.
Protein production
IPTG Induction and Extraction
jfjdfdjskjdshfjdhfjdjkdfbdbhdfhdgfhdbfdhfdg
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 580
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
1. Khairutdinov, B. I., Ermakova, E. A., Yusypovych, Y. M., Bessolicina, E. K., Tarasova, N. B., Toporkova, Y. Y., ... & Nesmelova, I. V. (2017). NMR structure, conformational dynamics, and biological activity of PsDef1 defensin from Pinus sylvestris. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1865(8), 1085-1094. doi:10.1016/j.bbapap.2017.05.012
2. Lee, J. E., Hofhaus, G., & Lisowsky, T. (2000). Erv1p from Saccharomyces cerevisiae is a FAD‐linked sulfhydryl oxidase. FEBS letters, 477(1-2), 62-66. doi: 10.1016/s0014-5793(00)01767-1
3. Kovalyova, V. A., Gout, I. T., Kyamova, R. G., Filonenko, V. V., & Gout, R. T. (2007). Cloning and analysis of defensin 1 cDNA from Scots pine. Biopolymers and Cell,23(5), 398-404. doi: http://dx.doi.org/10.7124/bc.000779
4. Veggiani, G., & de Marco, A. (2011). Improved quantitative and qualitative production of single-domain intrabodies mediated by the co-expression of Erv1p sulfhydryl oxidase. Protein expression and purification, 79(1), 111-114. doi: 10.1016/j.pep.2011.03.005
5. Hatahet, F., Nguyen, V. D., Salo, K. E., & Ruddock, L. W. (2010). Disruption of reducing pathways is not essential for efficient disulfide bond formation in the cytoplasm of E. coli. Microbial cell factories, 9(1), 67. doi: 10.1186/1475-2859-9-67
