Difference between revisions of "Part:BBa K2984019"
Chlamy Dima (Talk | contribs) |
|||
| Line 44: | Line 44: | ||
<img class="is-revealing" src="https://2019.igem.org/wiki/images/5/50/T--Humboldt_Berlin--YFP_fluorescence_difference_spectrum.png" alt="fluorescence difference spectrum" width="600" /> | <img class="is-revealing" src="https://2019.igem.org/wiki/images/5/50/T--Humboldt_Berlin--YFP_fluorescence_difference_spectrum.png" alt="fluorescence difference spectrum" width="600" /> | ||
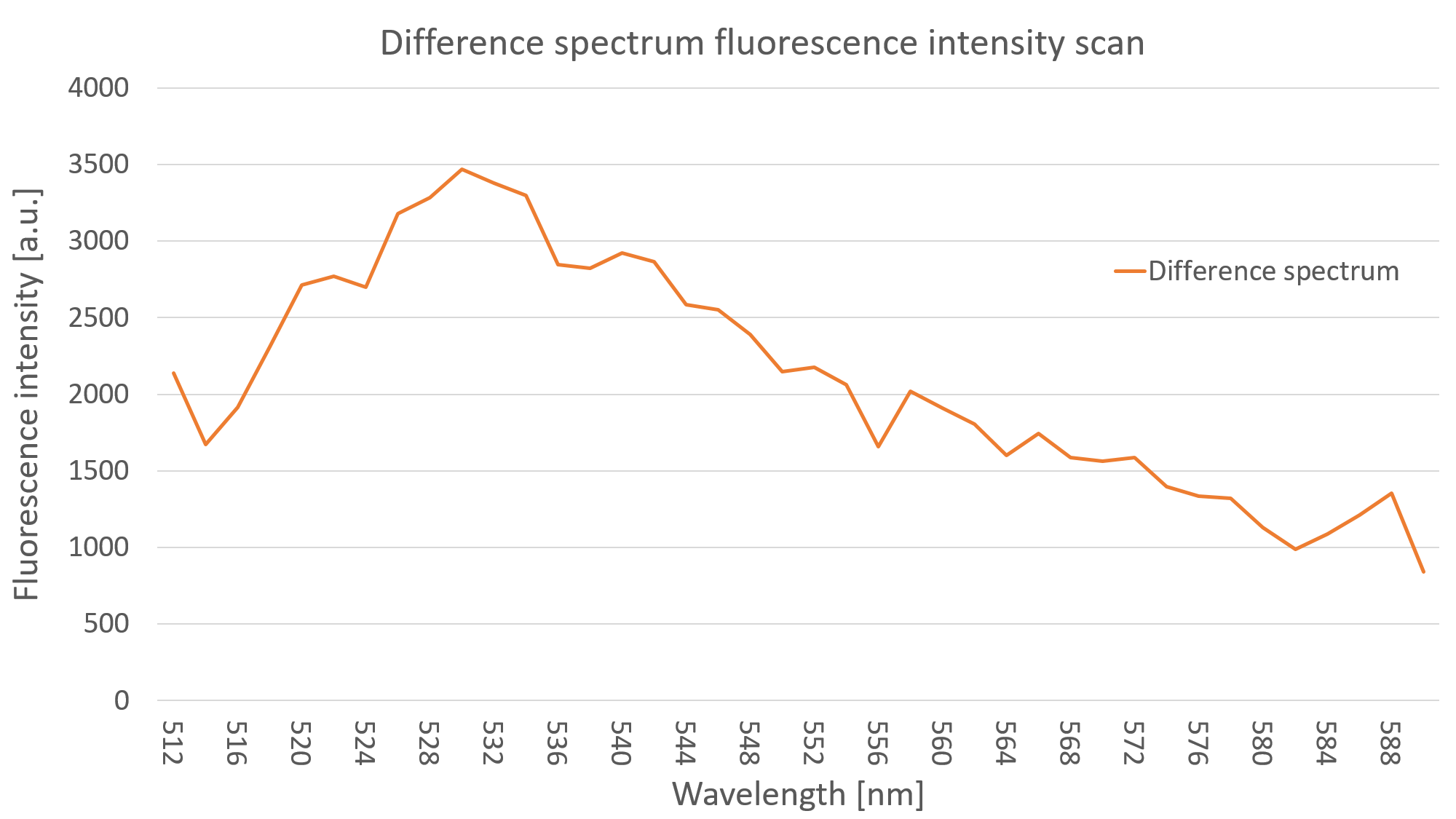
<figcaption>Fig. 3 - YFP emission spectrum of a C. reinhardtii clone with YFP</figcaption> | <figcaption>Fig. 3 - YFP emission spectrum of a C. reinhardtii clone with YFP</figcaption> | ||
| + | |||
| + | |||
| + | </html> | ||
| + | |||
| + | ===Psad Characterization - Light induced expression of YFP=== | ||
| + | <html> | ||
| + | <p> | ||
| + | As further characterization of the <a href=""> psad </a> promoter, we wanted to see if the part is influence by light. | ||
| + | Therefor we prepared three different sets of algae 3 days in advance. The first teo included <i> C.reinhardtii </i> algea which expressed our this composite part. the third one inlcuded our wt algea strain UVM 4. The first and the second were grown under synchronos light conditons, meaning they were exposed to light for 14 hours and to darkness for 10 hours. The second set | ||
| + | remained in darkness the hole time. All three were constantly shaked at speed of 110 round per minute while temperature was set to 21,4°C. The algea were grown in a black 96-well plate. After three days of growth we startet a time resolved fluorescence intensity measurement. We measured every 20 minutes for approximately hours. It is important to shake the plate with algae before each measurement to avoid the aggregation of algae on the bottom of the wells, which ultimately affects the measurement of the YFP. | ||
| + | |||
| + | Time resolved measurements are of great interest to examine dynamic processes in cells. Once we had achieved measurements of the fluorescence intensity and fluorescence spectrum of our YFP clones, we wanted to do time resolved measurements of the fluorescence intensity in our algae. The main objective of these measurements was the characterization of the promoter PsaD, which we used in most of our genetic constructs during the iGEM competition. Now that we knew how to measure the fluorescence intensity of our YFP expressing clones (Fig. 1), we could use the same protocol to measure time resolved fluorescence. We did a fluorescence intensity measurement every 20 minutes for approximately 40 hours. It is important to shake the plate with algae before each measurement to avoid the aggregation of algae on the bottom of the wells, which ultimately affects the measurement of the YFP. During our time resolved measurement we activated the expression of YFP with the PsaD promoter, which is light inducible. We wanted to quantify the light induction of PsaD by alternating between dark phases and light phases, which we successfully achieved | ||
| + | </p> | ||
| + | |||
<img class="is-revealing" src="https://2019.igem.org/wiki/images/e/e5/T--Humboldt_Berlin--Psad-YFP-syn%28FL_OD%29-1day.png" width="600" /> | <img class="is-revealing" src="https://2019.igem.org/wiki/images/e/e5/T--Humboldt_Berlin--Psad-YFP-syn%28FL_OD%29-1day.png" width="600" /> | ||
Revision as of 16:18, 17 October 2019
L1c-Psad-YFP-RbcS2, Expression of YFP through light-inducible promoter
This part is a part of the Chlamy-HUB-Collection. This Level 1 Construct is designed for comparison of locus dependent expression through fluorescence intensity measuerments. The Construct consist of the Psad promoter an coding sequence for the yellow fluorescent protein and the Rbcs2 terminator. The promoter is inducible by light.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 1046
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 1046
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 4
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 1046
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 1046
Illegal NgoMIV site found at 1523 - 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
This construct is designed for the expression of the yellow fluorescent protein (YFP) mVenus in C. reinhardtii. It is composed of the PsaD promoter, which stems from the photosystem one complex, the mVenus coding sequence and the terminator Rbcs2, taken from the Rubisco gene. This construct will make C. reinhardtii glow under a fluorescence microscope.
The YFP mVenus has an excitation peak at 515 nm and an emission peak at 528 nm (Kremers et al. 2006). With this construct we focused on the characterization of the light induction of PsaD. Nevertheless, there are many possible uses for this construct. For example, the construct can be used for the comparison of expression rates in dependency of the locus of transgene insertion.
Characterization
The first qualitative characterization we made was to check the mVenus fluorescence of C. reinhardtii that had been transformed with this construct under a fluorescence microscope.

After seeing fluorescence under the microscope, we proceeded to measure the fluorescence intensity of our clone under a plate reader (Fig. 2). Measuring the fluorescence intensity of YFP in C. reinhardtii can be quite challenging, due to the strong interaction of the algae with light (photo systems, chlorophyll, pigments and light antennae). You can find more information about how to measure this part on the measurements siteof the Humboldt Berlin team 2019.
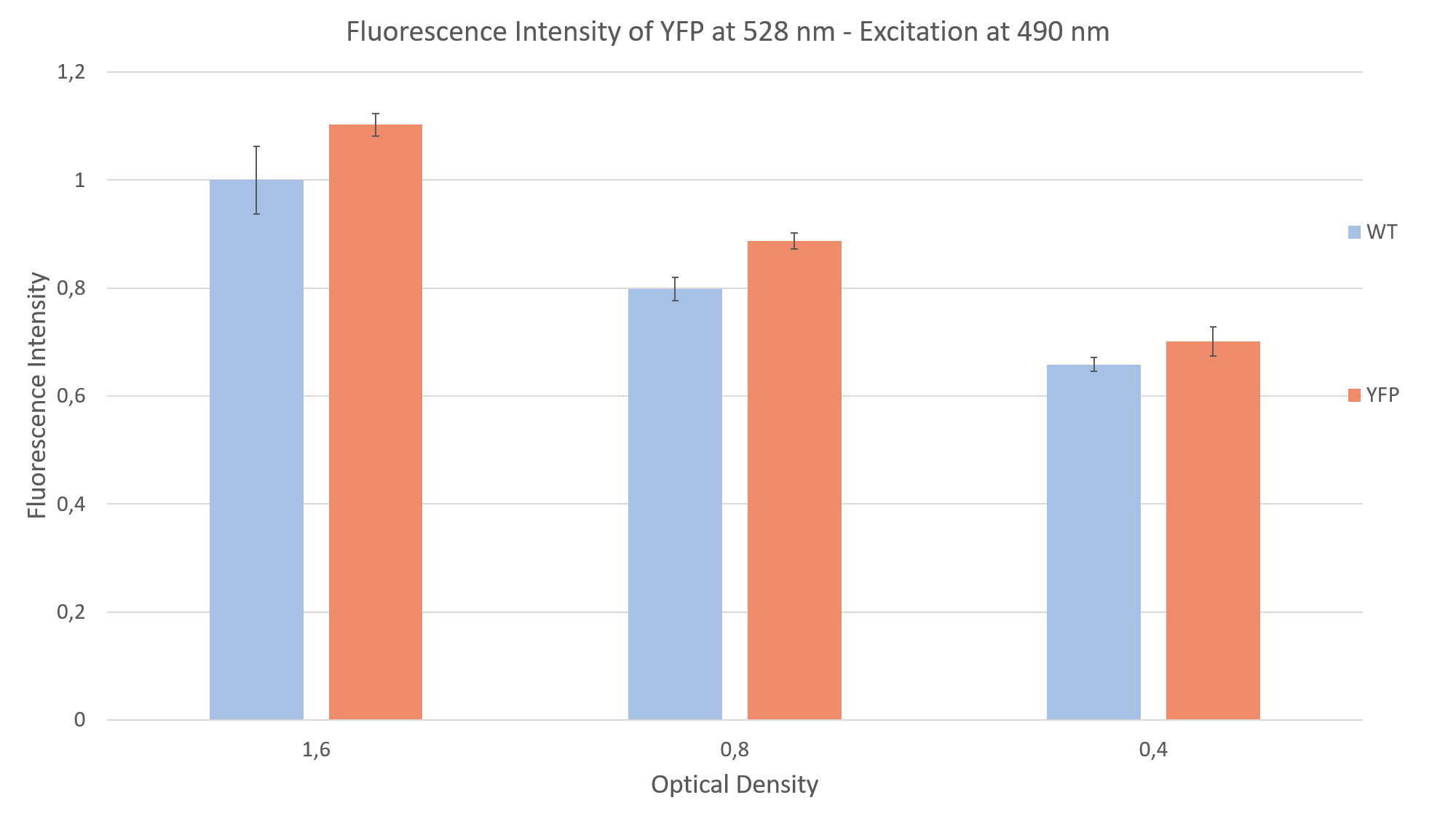
For the measurement we prepared YFP expressing clones and WT algae, to compare the fluorescence of both probes. This is imperative due to the strong autofluorescence of the algae. Additionally, we made sure to have the same optical density (cell concentration) in both YFP and WT probes. We did a sequential dilution in three steps with the probes and measured the fluorescence intensity for each probe. We excited at 490 nm and measured the emission at 528 nm. We were able to show that the fluorescence intensity of the YFP expressing clones was always above the autofluorescence of the wild type, as can be seen in Fig. 2.
After measuring the fluorescence intensity we also measured the emission spectrum of mVenus in C. reinhardtii by doing an emission scan of both YFP expressing clones and WT algae. By doing a difference spectrum of the fluorescence emission spectra of the mentioned probes, we received the emission spectrum of YFP, as can be seen on Fig. 3.
Psad Characterization - Light induced expression of YFP
As further characterization of the psad promoter, we wanted to see if the part is influence by light. Therefor we prepared three different sets of algae 3 days in advance. The first teo included C.reinhardtii algea which expressed our this composite part. the third one inlcuded our wt algea strain UVM 4. The first and the second were grown under synchronos light conditons, meaning they were exposed to light for 14 hours and to darkness for 10 hours. The second set remained in darkness the hole time. All three were constantly shaked at speed of 110 round per minute while temperature was set to 21,4°C. The algea were grown in a black 96-well plate. After three days of growth we startet a time resolved fluorescence intensity measurement. We measured every 20 minutes for approximately hours. It is important to shake the plate with algae before each measurement to avoid the aggregation of algae on the bottom of the wells, which ultimately affects the measurement of the YFP. Time resolved measurements are of great interest to examine dynamic processes in cells. Once we had achieved measurements of the fluorescence intensity and fluorescence spectrum of our YFP clones, we wanted to do time resolved measurements of the fluorescence intensity in our algae. The main objective of these measurements was the characterization of the promoter PsaD, which we used in most of our genetic constructs during the iGEM competition. Now that we knew how to measure the fluorescence intensity of our YFP expressing clones (Fig. 1), we could use the same protocol to measure time resolved fluorescence. We did a fluorescence intensity measurement every 20 minutes for approximately 40 hours. It is important to shake the plate with algae before each measurement to avoid the aggregation of algae on the bottom of the wells, which ultimately affects the measurement of the YFP. During our time resolved measurement we activated the expression of YFP with the PsaD promoter, which is light inducible. We wanted to quantify the light induction of PsaD by alternating between dark phases and light phases, which we successfully achieved



References
- Kremers, G. J., Goedhart, J., van Munster, E. B., & Gadella, T. W. (2006). Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET Förster radius. Biochemistry, 45(21), 6570-6580.
