Difference between revisions of "Part:BBa K864402"
| Line 25: | Line 25: | ||
</ul> | </ul> | ||
<br><br> | <br><br> | ||
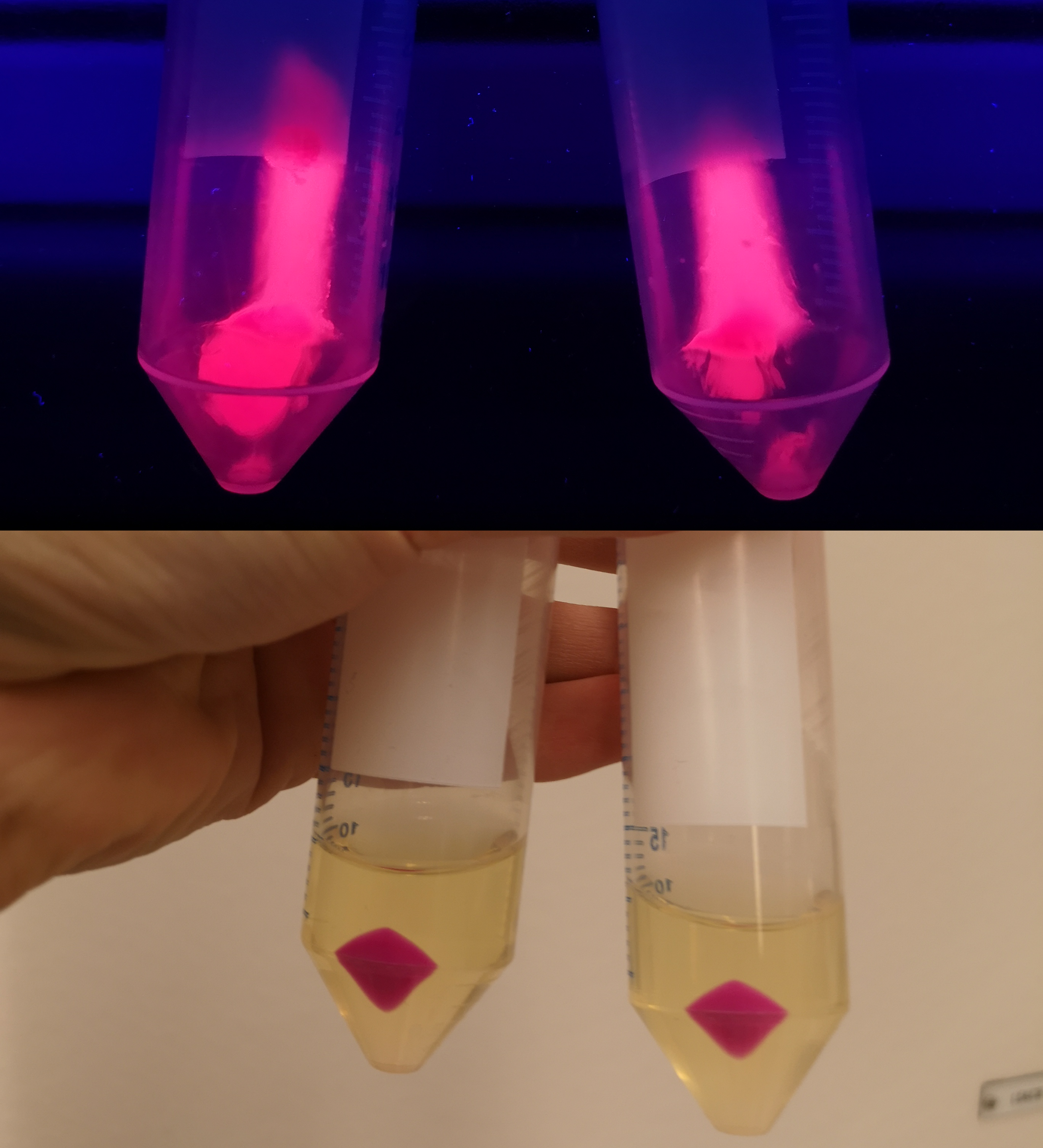
| − | <p> To verify eforRed's absorbance and emission, the construct was expressed in <i>E. coli </i> BL21 (DE3). The bacteria containing the constitutive promotor (pCons) was compared to a negative control ( | + | <p> To verify eforRed's absorbance and emission, the construct was expressed in <i>E. coli </i> BL21 (DE3). The bacteria containing the constitutive promotor (pCons) was compared to a negative control (<b>Figure 1</b>). Thereafter the eforRed expressing bacteria was centrifuged, resulting in a pellet with a burgundy color. (<b>Figure 1</b>, top-right corner). To demonstrate the fluorescence of eforRed, the pellet was placed on a UV-table emitting a wavelength of 302 nm, (<b>figure 1</b>, down-right corner), which exhibited a pink glowing colour. The culture tubes had a constant supply of oxygen by using cotton plugs which is important for the folding of the eforRed chromophore, therefore leading to increased expression.</p> |
<br><br> | <br><br> | ||
</html>[[Image:T--Linkoping_Sweden--pcons-eforred.jpeg|460px]]<html> | </html>[[Image:T--Linkoping_Sweden--pcons-eforred.jpeg|460px]]<html> | ||
| Line 33: | Line 33: | ||
<br><br> | <br><br> | ||
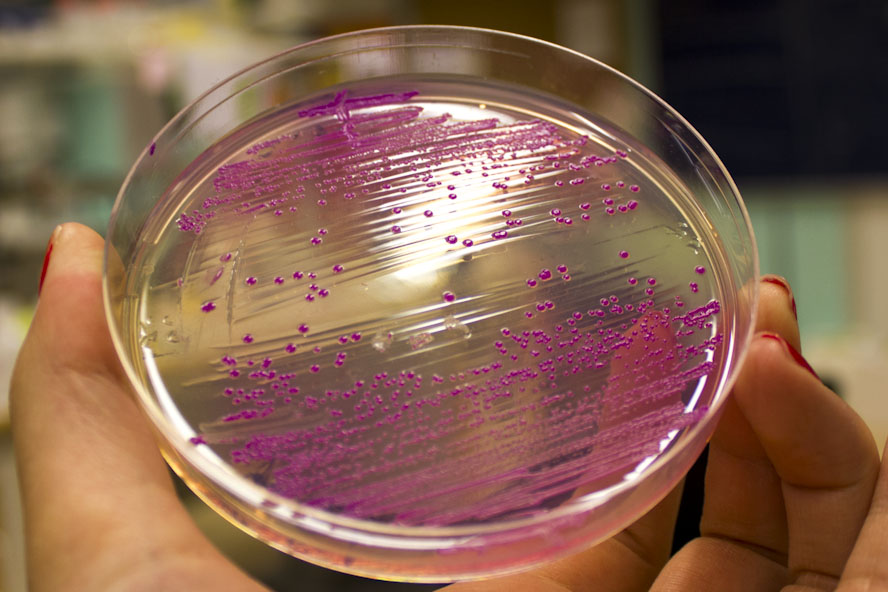
| − | Further characterization was performed in order to demonstrate the absorbance and fluorescence of E.coli BL21 (DE3) containing pCons eforRed. An eforRed culture was spread on an LB-agar plate containing 25 µg/ml chloramphenicol. The agar culture were photographed in visual light and on a UV-table emitting 302 nm (figure 2). The results were the same as above, in visual light (<b>Figure 2</b>, right) the cultures had a burgundy color and on the UV-table the bacteria exhibited a pink glowing colour (<b> Figure 2</b>, left). | + | Further characterization was performed in order to demonstrate the absorbance and fluorescence of <i>E. coli</i> BL21 (DE3) containing pCons eforRed. An eforRed culture was spread on an LB-agar plate containing 25 µg/ml chloramphenicol. The agar culture were photographed in visual light and on a UV-table emitting 302 nm (figure 2). The results were the same as above, in visual light (<b>Figure 2</b>, right) the cultures had a burgundy color and on the UV-table the bacteria exhibited a pink glowing colour (<b> Figure 2</b>, left). |
<br> | <br> | ||
<br><br> | <br><br> | ||
<div> | <div> | ||
| − | </html>[[Image:T--Linkoping_Sweden--eforred-agar-photo.png|700px|thumb|left|<div class="figurtext"style=font-size:80%;><i><b>Figure 2.</b> | + | </html>[[Image:T--Linkoping_Sweden--eforred-agar-photo.png|700px|thumb|left|<div class="figurtext"style=font-size:80%;><i><b>Figure 2.</b> <i> E.coli </i> BL21 (DE3) colonies presented in visual light (right side) and illuminated in 302 nm UV-light (left side). </i>]]<html> |
</div> | </div> | ||
| Line 44: | Line 44: | ||
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
<p> | <p> | ||
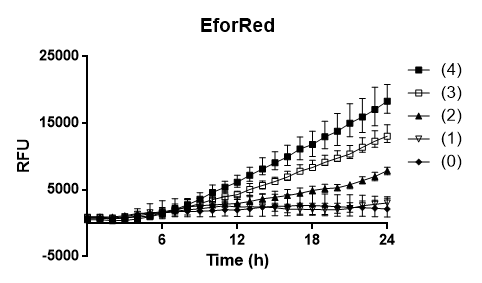
| − | To test the oxygen dependency of the protein production of eforRed in E.coli BL21 (DE3) , a platereading was conducted. | + | To test the oxygen dependency of the protein production of eforRed in <i> E. coli</i> BL21 (DE3) , a platereading was conducted. <i> E. coli</i> BL21 (DE3) was grown O.N. in 37 degrees Celsius to an 2.0 OD<sub>600</sub> and diluted to 0.49 OD<sub>600</sub>, 200 ul of the the bacteria was pipetted into 96-well plates with replicates of 4. Oxygen access was varied by piercing different numbers of holes (0,1,2,3 and 4) in the plastic film of the 96-well plate. The experiment showed that 4 holes in plastic film gave the highest protein yield and that the access to oxygen effects <I>E. colis BL21 (DE3) production of eforRed..</p> |
</div> | </div> | ||
<br> | <br> | ||
| Line 52: | Line 52: | ||
</div> | </div> | ||
<br><br> | <br><br> | ||
| − | <div class="liucontent" style=font-size:80%;><i><b>Figure 3.</b></i> To the left is a platereading E.coli BL21 (DE3) | + | <div class="liucontent" style=font-size:80%;><i><b>Figure 3.</b></i> To the left is a of platereading <i>E. coli </i> BL21 (DE3) expressing eforRed with varying access to oxygen. The holes were made in the plastic cover of a 96-well plate and the test was run for 16 hours in 37 degrees Celsius. The plate can be seen on the right pictures left side. The plate to the right is E.coli BL21 (DE3) with a different expression system used to test a strong green/yellow fluorescent protein. Both plates were illuminated in 302 nm UV-light.</i></div> |
<br><br> | <br><br> | ||
<br><br> | <br><br> | ||
<br><br> | <br><br> | ||
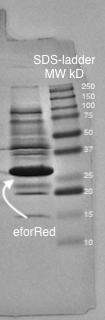
| − | A study of the molecular weight of | + | A study of the molecular weight of pCons eforRed expressed in <i>E. coli</i> BL21 (DE3) was done by sonicating the cells and performing a SDS-PAGE electrophoresis on the lysate (<b>Figure 4.</b>). Biorads "Precision Plus Protein Dual Color Standards" was used as the protein ladder in the electrophoresis.<br><br> |
</html>[[Image:T--Linkoping_Sweden--eforred_SDS-page.png|100px|left|]]<html> | </html>[[Image:T--Linkoping_Sweden--eforred_SDS-page.png|100px|left|]]<html> | ||
| − | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><div class="figurtext"style=font-size:80%;><b><i>Figure 4.</b> SDS-page of sonicated E.coli BL21 (DE3) lysate with | + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><div class="figurtext"style=font-size:80%;><b><i>Figure 4.</b> SDS-page of sonicated E.coli BL21 (DE3) lysate with pCons eforRed. Biorads "Precision Plus Protein Dual Color Standards" was used as the protein ladder. The visible band on the gel lies between 25 and 37 kD which corresponds to the molecular weight of eforRed which is 26.1 kDa.</i> |
</div> | </div> | ||
<br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br> | ||
Revision as of 09:25, 17 October 2019
J23110-B0034-eforRed
eforRed eforRed is previously described as BBa_K592012. We submitted a functionally active variant with J23110 and B0034.
Contribution
Group: Linkoping_Sweden iGEM 2019
Author: Andreas Holmqvist and Leo Juhlin
Summary:
In this contribution we characterized the visual absorbance and the fluorescence of this construct. We also tested the oxygen dependency of the protein expression in E. coli BL21(DE3) cells.
Documentation:
- the BBa_J23110Constitutive promotor
- the BBa_B0034Ribosome binding site
To verify eforRed's absorbance and emission, the construct was expressed in E. coli BL21 (DE3). The bacteria containing the constitutive promotor (pCons) was compared to a negative control (Figure 1). Thereafter the eforRed expressing bacteria was centrifuged, resulting in a pellet with a burgundy color. (Figure 1, top-right corner). To demonstrate the fluorescence of eforRed, the pellet was placed on a UV-table emitting a wavelength of 302 nm, (figure 1, down-right corner), which exhibited a pink glowing colour. The culture tubes had a constant supply of oxygen by using cotton plugs which is important for the folding of the eforRed chromophore, therefore leading to increased expression.

Further characterization was performed in order to demonstrate the absorbance and fluorescence of E. coli BL21 (DE3) containing pCons eforRed. An eforRed culture was spread on an LB-agar plate containing 25 µg/ml chloramphenicol. The agar culture were photographed in visual light and on a UV-table emitting 302 nm (figure 2). The results were the same as above, in visual light (Figure 2, right) the cultures had a burgundy color and on the UV-table the bacteria exhibited a pink glowing colour ( Figure 2, left).
To test the oxygen dependency of the protein production of eforRed in E. coli BL21 (DE3) , a platereading was conducted. E. coli BL21 (DE3) was grown O.N. in 37 degrees Celsius to an 2.0 OD600 and diluted to 0.49 OD600, 200 ul of the the bacteria was pipetted into 96-well plates with replicates of 4. Oxygen access was varied by piercing different numbers of holes (0,1,2,3 and 4) in the plastic film of the 96-well plate. The experiment showed that 4 holes in plastic film gave the highest protein yield and that the access to oxygen effects E. colis BL21 (DE3) production of eforRed..
A study of the molecular weight of pCons eforRed expressed in E. coli BL21 (DE3) was done by sonicating the cells and performing a SDS-PAGE electrophoresis on the lysate (Figure 4.). Biorads "Precision Plus Protein Dual Color Standards" was used as the protein ladder in the electrophoresis.
Sequence and Features