Difference between revisions of "Part:BBa K2984006"
Chlamy Dima (Talk | contribs) |
Chlamy Dima (Talk | contribs) |
||
| Line 14: | Line 14: | ||
For the gold medal criterium of improving an existing part, we submit this codon optimized version of the paromomycin resistance registered as BBa K2703008. By optimizing the codon usage of this part we expected a higher expression of the antibiotic resistance enzyme and therefore a higher rate of success when using this resistance gene for paromomycin resistance. Our characterization resulted in a confirmation of our hypothesis and a succesfull improvement of the BBa K2703008 part. | For the gold medal criterium of improving an existing part, we submit this codon optimized version of the paromomycin resistance registered as BBa K2703008. By optimizing the codon usage of this part we expected a higher expression of the antibiotic resistance enzyme and therefore a higher rate of success when using this resistance gene for paromomycin resistance. Our characterization resulted in a confirmation of our hypothesis and a succesfull improvement of the BBa K2703008 part. | ||
| − | <h3> | + | <h3>Biological background </h3> |
Paromomycin belongs to a group of aminoglycoside antibiotics such as neomycin or dibekacin. These aminoglycosides are capable of inhibiting the eukaryotic translation, by binding within the large and small subunit of the 80S ribosome. This property allows paromomycin to be used as a selection marker. The paromomycin resistance BBa_K2703008, registered by the Cambridge team 2016, works by allowing the expression of an enzyme which catalyses the transfer of the gamma-phosphate of ATP to the hydroxyl group in 3’ position of the paromomycin molecule and allows the carrier of the gene to develop a resistance to paromomycin. Some organisms exhibit a higher expression of transgene enzymes when the genetic code is codon optimized for the specific organism. One of these organisms is the alga Chlamydomonas reinhardtii. With the following part, we propose a codon optimized version of the paromomycin resistance for use in Chlamydomonas reinhardtii. This part is optimal for paromomycin screening when using C. reinhardtii | Paromomycin belongs to a group of aminoglycoside antibiotics such as neomycin or dibekacin. These aminoglycosides are capable of inhibiting the eukaryotic translation, by binding within the large and small subunit of the 80S ribosome. This property allows paromomycin to be used as a selection marker. The paromomycin resistance BBa_K2703008, registered by the Cambridge team 2016, works by allowing the expression of an enzyme which catalyses the transfer of the gamma-phosphate of ATP to the hydroxyl group in 3’ position of the paromomycin molecule and allows the carrier of the gene to develop a resistance to paromomycin. Some organisms exhibit a higher expression of transgene enzymes when the genetic code is codon optimized for the specific organism. One of these organisms is the alga Chlamydomonas reinhardtii. With the following part, we propose a codon optimized version of the paromomycin resistance for use in Chlamydomonas reinhardtii. This part is optimal for paromomycin screening when using C. reinhardtii | ||
| Line 20: | Line 20: | ||
=='''Characterization'''== | =='''Characterization'''== | ||
<html> | <html> | ||
| + | <h3> Methods </h3> | ||
| + | |||
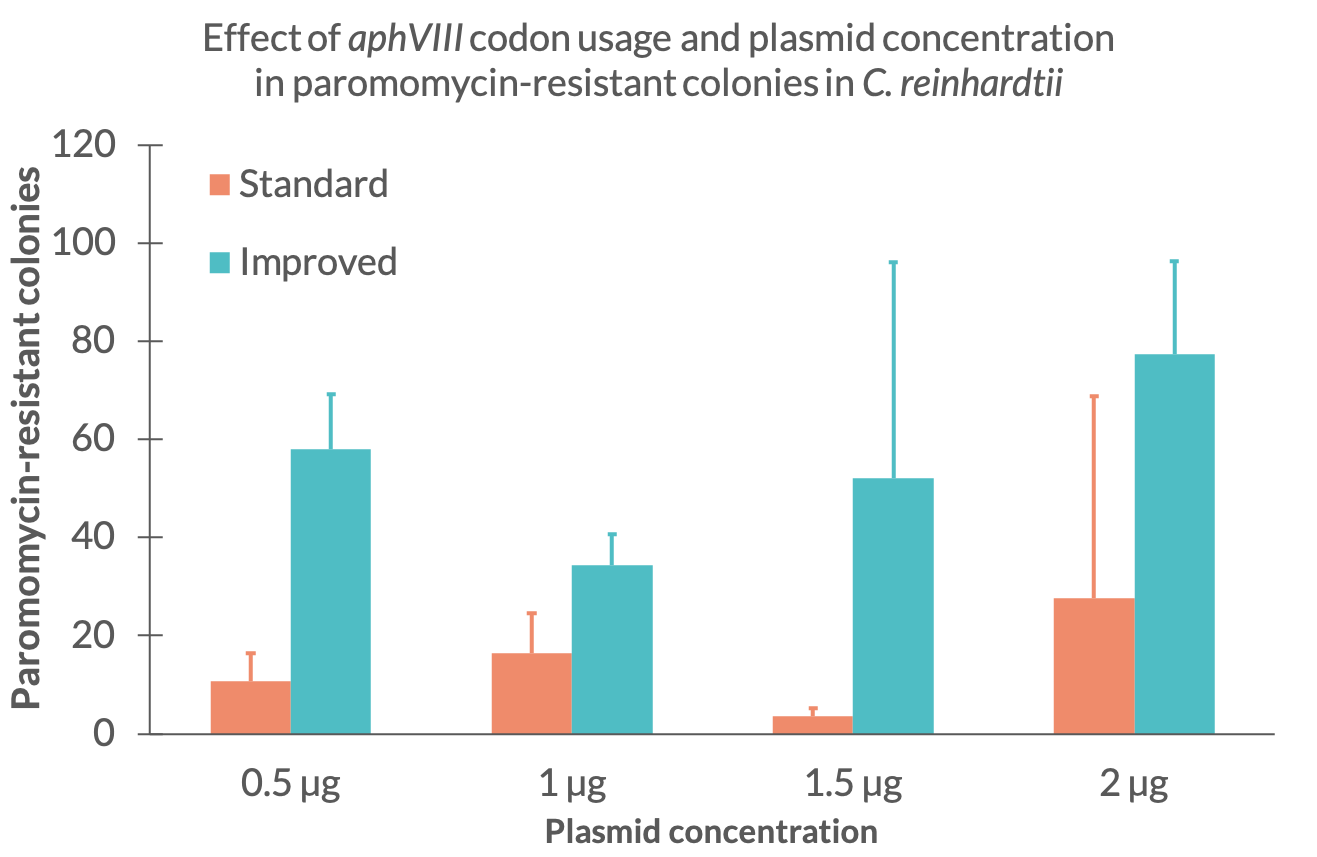
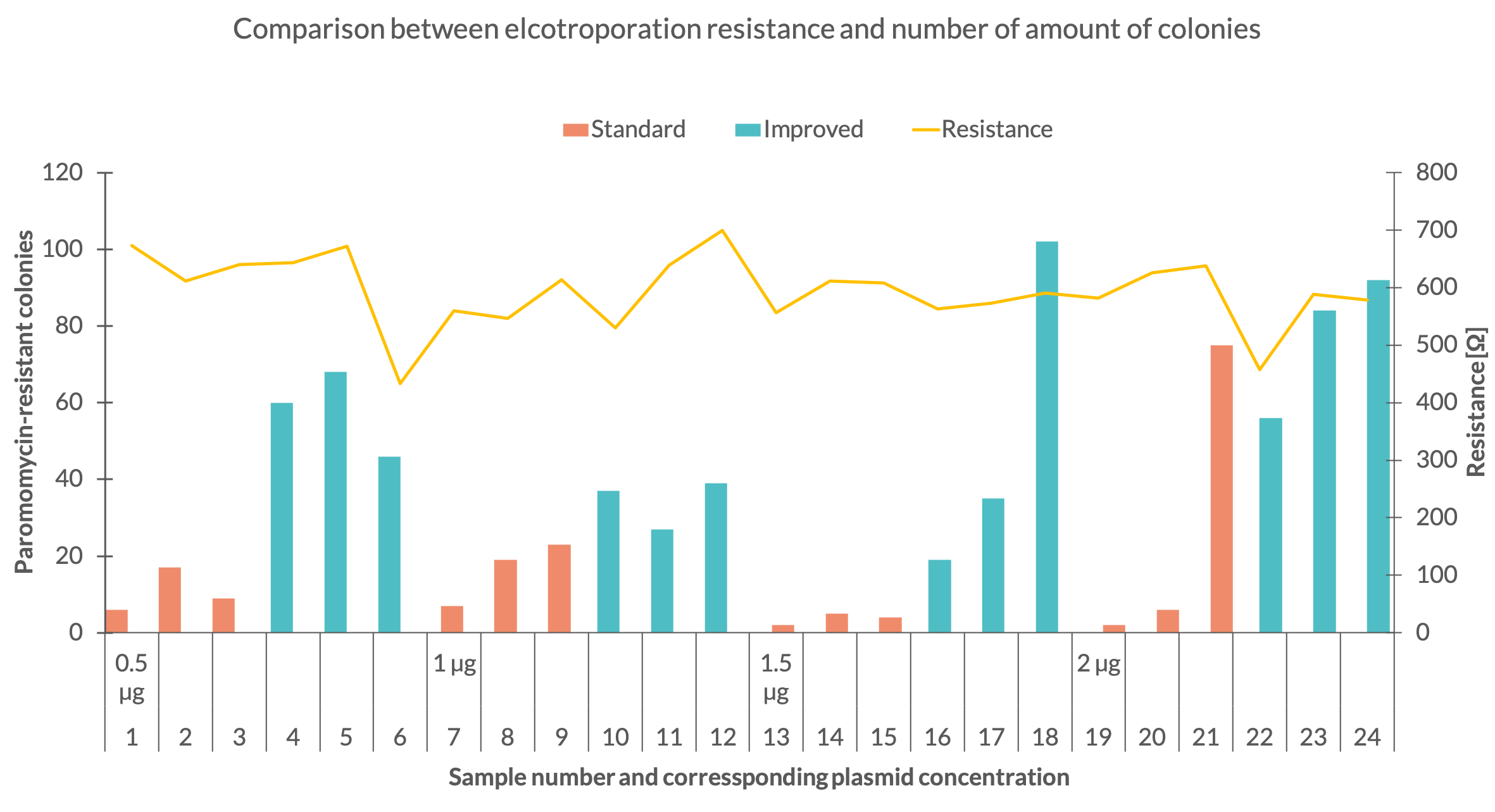
| + | To see if the expression of the aminoglycoside 3’-phosphotransferase was increased, we performed several electroporations to transform C. reinhardtii with the improved paromomycin resistance. We used the C.reinhardtii strain UVM 4 since it is a strain designed to express transgene constructs (Neupert et al. 2009). We transformed the two paromomycin constructs with standard and improved codon usage starting with 0,5 µg DNA per electroporation sample and ascended with 0,5 µg steps up to 2 µg. For each construct and DNA mass we did three electroporations. The electroporation electrical resistance was measured for each sample. After resuspension and one day recovery in TAP medium, all samples were plated on TAP-agar plates containing a paromomycin concentration of 10 µM. After two weeks of growth, colonies corresponding to each sample were counted. Each colony of C.reinhardtii represents a successful transformation of the resistance and indicates the expression of the aminoglycoside 3’-phosphotransferase. By counting the amount of colonies on the plates, we could determine which construct and at which DNA mass at the time of transformation worked best. | ||
| + | |||
| + | <h3> Results </h3> | ||
| + | |||
| + | First, we counted all the colonies of <i>C. reinhardtii</i> that were transformed with the standard and improved resistance. In Fig. 1 the results of the total colony count can be seen. | ||
<figure> | <figure> | ||
<img src="https://2019.igem.org/wiki/images/a/a7/T--Humboldt_Berlin--Colony_Amount_Total.png" alt="colonies_total" width="500"> | <img src="https://2019.igem.org/wiki/images/a/a7/T--Humboldt_Berlin--Colony_Amount_Total.png" alt="colonies_total" width="500"> | ||
| − | <figcaption>Fig.1 - </figcaption> | + | <figcaption>Fig.1 - Total amount of colonies counted for the standard (blue) and improved (organge) resistance</figcaption> |
</figure> | </figure> | ||
| + | |||
| + | |||
<figure> | <figure> | ||
| Line 35: | Line 44: | ||
<figcaption>Fig 3. - </figcaption> | <figcaption>Fig 3. - </figcaption> | ||
</figure> | </figure> | ||
| + | |||
| + | =='''References'''== | ||
| + | [1] Neupert, J., Karcher, D., & Bock, R. (2009). Generation of Chlamydomonas strains that efficiently express nuclear transgenes. The Plant Journal, 57(6), 1140-1150. | ||
| + | |||
| + | [2] Sizova, I., Fuhrmann, M., & Hegemann, P. (2001). A Streptomyces rimosusaphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene, 277(1-2), 221-229. | ||
Revision as of 12:28, 13 October 2019
Codon-Usage-Optimized Paromomycin Resistance For Use in Chlamydomonas reinhardtii
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 298
Illegal NotI site found at 122 - 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 13
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 401
- 1000COMPATIBLE WITH RFC[1000]
Introduction
For the gold medal criterium of improving an existing part, we submit this codon optimized version of the paromomycin resistance registered as BBa K2703008. By optimizing the codon usage of this part we expected a higher expression of the antibiotic resistance enzyme and therefore a higher rate of success when using this resistance gene for paromomycin resistance. Our characterization resulted in a confirmation of our hypothesis and a succesfull improvement of the BBa K2703008 part.
Biological background
Paromomycin belongs to a group of aminoglycoside antibiotics such as neomycin or dibekacin. These aminoglycosides are capable of inhibiting the eukaryotic translation, by binding within the large and small subunit of the 80S ribosome. This property allows paromomycin to be used as a selection marker. The paromomycin resistance BBa_K2703008, registered by the Cambridge team 2016, works by allowing the expression of an enzyme which catalyses the transfer of the gamma-phosphate of ATP to the hydroxyl group in 3’ position of the paromomycin molecule and allows the carrier of the gene to develop a resistance to paromomycin. Some organisms exhibit a higher expression of transgene enzymes when the genetic code is codon optimized for the specific organism. One of these organisms is the alga Chlamydomonas reinhardtii. With the following part, we propose a codon optimized version of the paromomycin resistance for use in Chlamydomonas reinhardtii. This part is optimal for paromomycin screening when using C. reinhardtii
Characterization
Methods
To see if the expression of the aminoglycoside 3’-phosphotransferase was increased, we performed several electroporations to transform C. reinhardtii with the improved paromomycin resistance. We used the C.reinhardtii strain UVM 4 since it is a strain designed to express transgene constructs (Neupert et al. 2009). We transformed the two paromomycin constructs with standard and improved codon usage starting with 0,5 µg DNA per electroporation sample and ascended with 0,5 µg steps up to 2 µg. For each construct and DNA mass we did three electroporations. The electroporation electrical resistance was measured for each sample. After resuspension and one day recovery in TAP medium, all samples were plated on TAP-agar plates containing a paromomycin concentration of 10 µM. After two weeks of growth, colonies corresponding to each sample were counted. Each colony of C.reinhardtii represents a successful transformation of the resistance and indicates the expression of the aminoglycoside 3’-phosphotransferase. By counting the amount of colonies on the plates, we could determine which construct and at which DNA mass at the time of transformation worked best.Results
First, we counted all the colonies of C. reinhardtii that were transformed with the standard and improved resistance. In Fig. 1 the results of the total colony count can be seen.