Difference between revisions of "Part:BBa K1758344"
(Created page with "<partinfo>BBa_K1758344 short</partinfo> mercury induceble promoter under the control of a T7 promoter with 5´untranslated region and sfGFP for detection ===Usage and Biology==...") |
|||
| (7 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
<partinfo>BBa_K1758344 short</partinfo> | <partinfo>BBa_K1758344 short</partinfo> | ||
| − | + | Mercury inducible promoter under the control of a T7 promoter with 5´untranslated region and sfGFP for detection | |
===Usage and Biology=== | ===Usage and Biology=== | ||
<html> | <html> | ||
| − | <p>For the in vitro characterization we used a cell extract out of cells which contain the | + | <p>The promoter <i>PmerT</i> is regulated by the MerR, which binds Hg2+-ions. Similar to the former sensors we added sfGFP for detection via fluorescence. It is based on BBa_K346001 designed by team Peking 2010. For the in vitro characterization we used a cell extract out of cells which contain the plasmid (<a href="https://parts.igem.org/Part:BBa_K1758340" target="_blank"> BBa_K1758340</a>). In addition to that we added plasmid DNA of the mercury specific promoter merT with 5’UTR-sfGFP under the control of T7-promoter (<a href="https://parts.igem.org/Part:BBa_K1758344" target="_blank"> BBa_K1758344</a>)to the cell extract. The T7 promoter is needed to get a better expression. </p> |
</html> | </html> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
| − | <partinfo> | + | <partinfo>BBa_K1758344 SequenceAndFeatures</partinfo> |
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| − | <partinfo> | + | <partinfo>BBa_K1758344 parameters</partinfo> |
<!-- --> | <!-- --> | ||
| + | |||
| + | <html> | ||
| + | <h2><i>in vitro</i></h2> | ||
| + | <p>For the characterization of the mercury sensor with CFPS we used parts differing from that we used in the <i>in vivo</i> characterization. For the <i>in vitro</i> characterization we used a cell extract out of cells, which contained the plasmid (<a href="https://parts.igem.org/Part:BBa_K1758340" target="_blank"> BBa_K1758340</a>)(figure 5). In addition, we added plasmid DNA to the cell extract. This plasmid consisted of the mercury specific promoter <i>pmerT</i> with 5’-UTR-sfGFP. The entire sequence was placed under the control of of T7 promoter (<a href="https://parts.igem.org/Part:BBa_K1758344" target="_blank"> BBa_K1758344</a>)(figure 6). The T7-promoter is needed to get a better fluorescence expression.</p> | ||
| + | |||
| + | <div class="row"> | ||
| + | <div class="col-md-6 text-center" style="margin-bottom: 50px"> <figure> | ||
| + | <a href=" https://static.igem.org/mediawiki/2015/3/3c/Bielefeld-CeBiTec_in_vitro_merR-part.jpeg" data-lightbox="heavymetals" data-title=" Figure 5: To produce the cell extract for <i>in vitro</i> characterization a construct (BBa_K1758340 ) with chromium repressor under the control of a constitutive promoter and strong RBS. " alt="repressor construct used for in vivo characterization."><img src=" https://static.igem.org/mediawiki/2015/3/3c/Bielefeld-CeBiTec_in_vitro_merR-part.jpeg" alt="repressor construct used for in vitro characterisation" style="width:500px"></a> <figcaption>Figure 5: To produce the cell extract for <i>in vitro</i> characterization a construct (<a href="https://parts.igem.org/Part:BBa_K175840" target="_blank">BBa_K175840</a>) with chromium repressor under the control of a constitutive promoter and strong RBS (BBa_K608002) is needed. </figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <div class="col-md-6 text-center" style="margin-bottom: 50px"> <figure> | ||
| + | <a href=" https://static.igem.org/mediawiki/2015/e/e2/Bielefeld-CebiTec_in_vitro_T7-merT-UTR-sfGFP.jpeg " data-lightbox="heavymetals" data-title="T7-PmerT-UTR-sfGFP used for<i>in vitro</i> characterization." https://static.igem.org/mediawiki/2015/e/e2/Bielefeld-CebiTec_in_vitro_T7-merT-UTR-sfGFP.jpeg " alt="promoter construct used for in vivo characterization."><img src=" https://static.igem.org/mediawiki/2015/e/e2/Bielefeld-CebiTec_in_vitro_T7-merT-UTR-sfGFP.jpeg" alt="promoter construct used for in vivo characterisation " style="width:500px"></a> <figcaption>T7-<i>PmerT</i>-UTR-sfGFP <a href="https://parts.igem.org/Part:BBa_K1758344" target="_blank"> BBa_K175844</a> used for<i>in vitro</i> characterization.</figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <figure style="width: 600px"> | ||
| + | <img src="https://static.igem.org/mediawiki/2015/b/b9/Bielefeld-CeBiTec_Influence_of_mercury_on_the_cell_extract.jpeg" width="100%"> | ||
| + | <figcaption>Figure 7: Influence of different mercury concentrations on our crude cell extract. Error bars represent the standard deviation of three biological replicates.</figcaption> | ||
| + | </figure> | ||
| + | |||
| + | <figure> | ||
| + | <img src="https://static.igem.org/mediawiki/2015/7/7f/Bielefeld-CeBiTec_induction_mercury_in_merR_cell-extract.jpeg" width="100%"</a> | ||
| + | <figcaption>Figure 8: Mercury specific cell extract made from E. coli cells, which have already expressed the activator before cell extract production. Induction of mercury inducible promoter without T7 in front of the operator site with different mercury concentrations. Error bars represent the standard deviation of three biological replicates. </figcaption> | ||
| + | </figure> | ||
| + | |||
| + | <figure> | ||
| + | <img src="https://static.igem.org/mediawiki/2015/f/f9/Bielefeld-CeBiTec_correction_induction_mercury_in_merR_cell-extract.jpeg" width="100%"> | ||
| + | <figcaption>Figure 9: Mercury specific cell extract made from <i>E. coli</i> cells, which have already expressed the activator before cell extract production. Induction of mercury inducible promoter without T7 in front of the operator site with different mercury concentrations. Error bars represent the standard deviation of three biological replicates. </figcaption> | ||
| + | </figure> | ||
| + | |||
| + | |||
| + | <p><i>In vitro</i> this sensor showed good results. The fluorescence level was high at low concentrations. Additionally, it showed that the expression level at 6 µg/L (Guideline of WHO for Mercury) reached the maximal signal. This result indicated the potential for measurement of concentrations under 6 µg/L.To confirm this hypothesis, it takes more experiments and tests with lower concentrations. Due to the high expression of sfGFP at low concentrations and the same expression level at different concentrations, it is not possible to quantify mercury with CFPS analyses . , Our model predicted this observation. During the measurement we noticed that the heavy metals have negative influences on the cell extract. Because of this fact, we used a correction factor, which resulted from the heavy metals influence on the CFPS system. This already optimized sensor showed the high potential of optimized sensors in CFPS.</p> | ||
| + | |||
| + | <b>References</b> | ||
| + | <p>iGEM Team Peking 2010</p> | ||
| + | <p> Brown, Nigel L.; Stoyanov, , Jivko V.;Kidd,Stephen P.;Hobman; Jon L. (2003): The MerR family of transcriptional regulators. In FEMS Microbiology Reviews, 27 ( 2) pp.145-163.</p> | ||
| + | |||
| + | |||
| + | </html> | ||
Latest revision as of 13:33, 7 March 2019
Mercury responsive promoter with T7-promoter and UTR-sfGFP
Mercury inducible promoter under the control of a T7 promoter with 5´untranslated region and sfGFP for detection
Usage and Biology
The promoter PmerT is regulated by the MerR, which binds Hg2+-ions. Similar to the former sensors we added sfGFP for detection via fluorescence. It is based on BBa_K346001 designed by team Peking 2010. For the in vitro characterization we used a cell extract out of cells which contain the plasmid ( BBa_K1758340). In addition to that we added plasmid DNA of the mercury specific promoter merT with 5’UTR-sfGFP under the control of T7-promoter ( BBa_K1758344)to the cell extract. The T7 promoter is needed to get a better expression.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 145
in vitro
For the characterization of the mercury sensor with CFPS we used parts differing from that we used in the in vivo characterization. For the in vitro characterization we used a cell extract out of cells, which contained the plasmid ( BBa_K1758340)(figure 5). In addition, we added plasmid DNA to the cell extract. This plasmid consisted of the mercury specific promoter pmerT with 5’-UTR-sfGFP. The entire sequence was placed under the control of of T7 promoter ( BBa_K1758344)(figure 6). The T7-promoter is needed to get a better fluorescence expression.




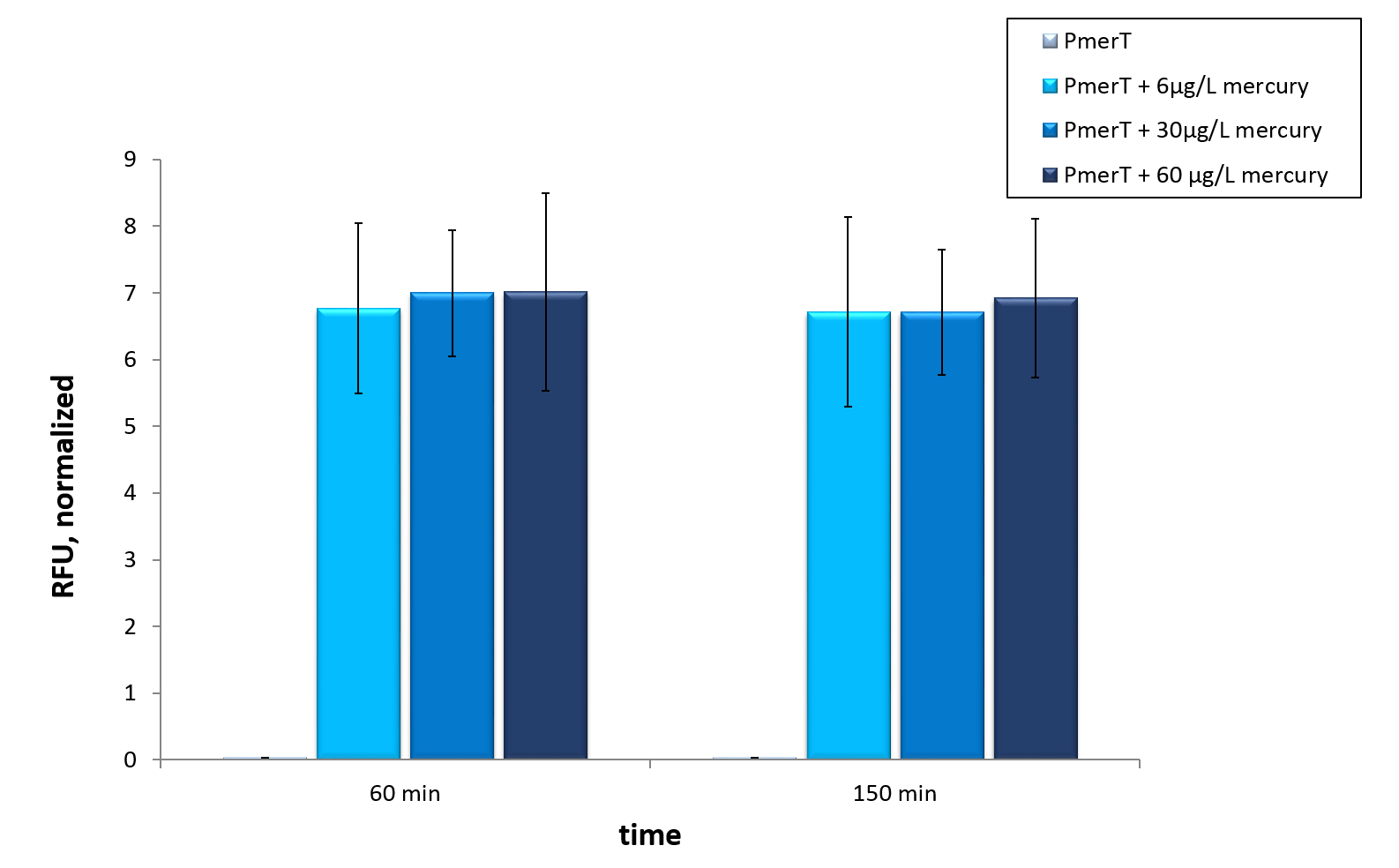
In vitro this sensor showed good results. The fluorescence level was high at low concentrations. Additionally, it showed that the expression level at 6 µg/L (Guideline of WHO for Mercury) reached the maximal signal. This result indicated the potential for measurement of concentrations under 6 µg/L.To confirm this hypothesis, it takes more experiments and tests with lower concentrations. Due to the high expression of sfGFP at low concentrations and the same expression level at different concentrations, it is not possible to quantify mercury with CFPS analyses . , Our model predicted this observation. During the measurement we noticed that the heavy metals have negative influences on the cell extract. Because of this fact, we used a correction factor, which resulted from the heavy metals influence on the CFPS system. This already optimized sensor showed the high potential of optimized sensors in CFPS.
ReferencesiGEM Team Peking 2010
Brown, Nigel L.; Stoyanov, , Jivko V.;Kidd,Stephen P.;Hobman; Jon L. (2003): The MerR family of transcriptional regulators. In FEMS Microbiology Reviews, 27 ( 2) pp.145-163.
