Difference between revisions of "Part:BBa K2558004"
| (2 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K2558004 short</partinfo> | <partinfo>BBa_K2558004 short</partinfo> | ||
| − | Tac promotor (trp/lacUV5 hybrid) | + | Tac promotor (trp/lacUV5 hybrid) is a hybrid between Trp and lacUV5 promoters. It is a kind of strong E. coli promotor and is inducible with IPTG. |
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | In order to investigate how lacI dosage affect IPTG induction we used Anderson promotor J23100, J23110 and J23114 to design three constitutive lacI generator of different intensity. The three lacI generator were then ligated with Ptac controlled reporter sfGFP to make three IPTG induction devices (https://parts.igem.org/Part:BBa_K2558203, https://parts.igem.org/Part:BBa_K2558204, https://parts.igem.org/Part:BBa_K2558205). By measuring sfGFP fluorescence we tested how these devices react to IPTG. | ||
| + | <!-- --> | ||
| + | ===Results=== | ||
| + | With high level of lacI expression (https://parts.igem.org/Part:BBa_K2558203), sfGFP fluorescence has almost no response to IPTG induction. Weak lacI expression (https://parts.igem.org/Part:BBa_K2558205) has the most significant IPTG induced sfGFP expression. With medium lacI concentration (https://parts.igem.org/Part:BBa_K2558204), the induction efficiency lies in between. Therefore, the result proves that high level of lacI expression severely decrease IPTG induction efficiency [1]. Furthermore, IPTG concentration can affect the regulation part performance. The figure shows that without IPTG the sfGFP florescence intensity will be at floor level. After IPTG addition, fluorescence signal immediately begins to climb, forming a peak at five hours after induction, then sfGFP florescence intensity will decrease and maintain at a lower level afterwards. IPTG concentration does not significantly affect the height of the peak or the expression level after the peak, but the peak width and expression stability of the system. Figures indicate that 5-10 mM IPTG has the most stable induction results. | ||
| + | <br/><li style="display: inline-block;"> [[File:T--Tsinghua-IPTGsfGFPresults-WithoutDescription.png|thumb|center|800px|'''Figure.1. The effect of varied lacI-LVA (BBa_C0011) promotor strength on IPTG induction of Tac promotor (BBa_K2558004).''' Relative fluorescent intensity is fluorescence per OD600 standardized with fluorescence per OD600 value of each test group at Time=0, IPTG=0. The promotors we used are from the Anderson collection: BBa_J23100 for strong lacI-LVA expression (pink), BBa_J23110 for medium expression (green), BBa_J23114 for weak expression (orange).]] | ||
<!-- --> | <!-- --> | ||
| − | + | ===Protocol=== | |
| − | + | # one Transform the plasmids into E. coli BL21(DE3) or DH5α. | |
| + | # two Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 5ml M9 medium with ampicillin at 100 ng/µl. Incubate for 6-8 h at 37℃ in a shaker. | ||
| + | # three Measure OD600 of the culture medium with photometer. Dilute the culture medium until OD600 reaches 0.6. | ||
| + | # four Add 100 µl bacteria culture medium into a sterile 96-well plate. Add IPTG to final concentrations of 0, 1, 5, 10, 20 mM. Fresh M9 medium serves as blank control. Positive control is colony constantly expressing sfGFP and negative control is colony without sfGFP expression. Place the 96-well plate into an automatic microplate reader. Incubate at 16℃ overnight and record the fluorometric value at 510 nm and OD600 for each well every 30 minutes. | ||
| + | # five Each group is repeated for at least 3 times. | ||
| + | ===Reference=== | ||
| + | [1] Szabolcs Semsey, Sandeep Krishna. "The effect of LacI autoregulation on the performance of the lactose utilization system in Escherichia coli" Nucleic Acids Res 2013 Jul; 41(13): 6381–6390 | ||
| + | |||
| + | ====Sequence and Features==== | ||
| + | <partinfo>BBa_K2558204 SequenceAndFeatures</partinfo> | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 14:20, 17 October 2018
Tac promotor (trp/lacUV5 hybrid)
Tac promotor (trp/lacUV5 hybrid) is a hybrid between Trp and lacUV5 promoters. It is a kind of strong E. coli promotor and is inducible with IPTG.
Usage and Biology
In order to investigate how lacI dosage affect IPTG induction we used Anderson promotor J23100, J23110 and J23114 to design three constitutive lacI generator of different intensity. The three lacI generator were then ligated with Ptac controlled reporter sfGFP to make three IPTG induction devices (https://parts.igem.org/Part:BBa_K2558203, https://parts.igem.org/Part:BBa_K2558204, https://parts.igem.org/Part:BBa_K2558205). By measuring sfGFP fluorescence we tested how these devices react to IPTG.
Results
With high level of lacI expression (https://parts.igem.org/Part:BBa_K2558203), sfGFP fluorescence has almost no response to IPTG induction. Weak lacI expression (https://parts.igem.org/Part:BBa_K2558205) has the most significant IPTG induced sfGFP expression. With medium lacI concentration (https://parts.igem.org/Part:BBa_K2558204), the induction efficiency lies in between. Therefore, the result proves that high level of lacI expression severely decrease IPTG induction efficiency [1]. Furthermore, IPTG concentration can affect the regulation part performance. The figure shows that without IPTG the sfGFP florescence intensity will be at floor level. After IPTG addition, fluorescence signal immediately begins to climb, forming a peak at five hours after induction, then sfGFP florescence intensity will decrease and maintain at a lower level afterwards. IPTG concentration does not significantly affect the height of the peak or the expression level after the peak, but the peak width and expression stability of the system. Figures indicate that 5-10 mM IPTG has the most stable induction results.
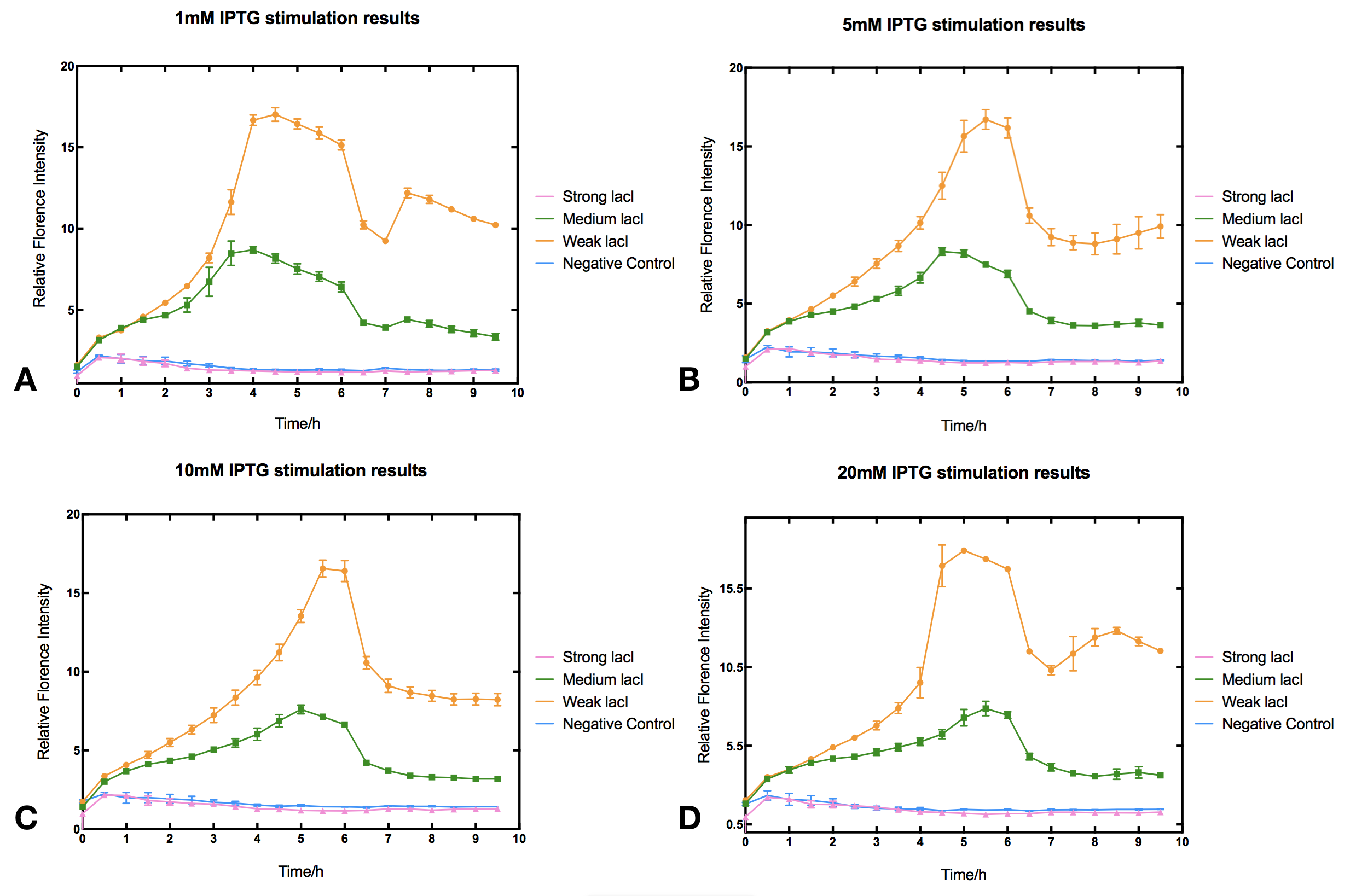
Protocol
- one Transform the plasmids into E. coli BL21(DE3) or DH5α.
- two Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 5ml M9 medium with ampicillin at 100 ng/µl. Incubate for 6-8 h at 37℃ in a shaker.
- three Measure OD600 of the culture medium with photometer. Dilute the culture medium until OD600 reaches 0.6.
- four Add 100 µl bacteria culture medium into a sterile 96-well plate. Add IPTG to final concentrations of 0, 1, 5, 10, 20 mM. Fresh M9 medium serves as blank control. Positive control is colony constantly expressing sfGFP and negative control is colony without sfGFP expression. Place the 96-well plate into an automatic microplate reader. Incubate at 16℃ overnight and record the fluorometric value at 510 nm and OD600 for each well every 30 minutes.
- five Each group is repeated for at least 3 times.
Reference
[1] Szabolcs Semsey, Sandeep Krishna. "The effect of LacI autoregulation on the performance of the lactose utilization system in Escherichia coli" Nucleic Acids Res 2013 Jul; 41(13): 6381–6390
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1073
Illegal NheI site found at 1096 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 2277
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 176
