Difference between revisions of "Part:BBa I746909:Experience"
| Line 13: | Line 13: | ||
</html> | </html> | ||
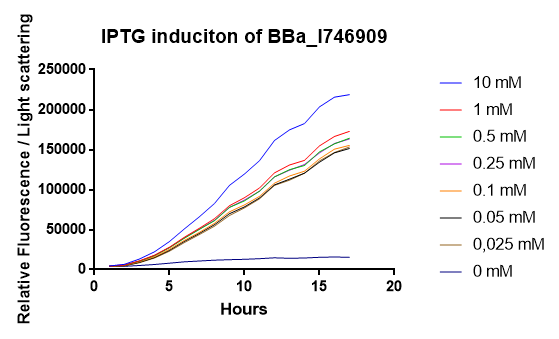
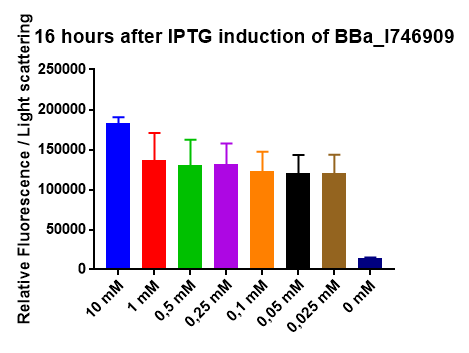
The IPTG induction of BBa_I746909 was validated by expressing the protein in E. coli. Fluorescent was measured over 16 hours after IPTG induction and the bacteria was incubated at 37 ͦC. Excitation was measured at 488 nm and emission at 510 nm. The optical density was measured at 600 nm. Different induction concentration of IPTG was used to stimulate the expression. Experiments where conducted in an infinite M1000 PRO plate reader. Graphs show the mean values of fluorescents per bacteria of 6 biological replicates. The error bars represent the 95 % confidence interval. Results seem to indicate that 10 mM IPTG will give the most effective response, that the IPTG promoter is very sensitive. We make that statement from the fact that our smallest induction concentration(0,025mM) induced almost the same amount of fluorescence as our next strongest induction concentration (1mM). | The IPTG induction of BBa_I746909 was validated by expressing the protein in E. coli. Fluorescent was measured over 16 hours after IPTG induction and the bacteria was incubated at 37 ͦC. Excitation was measured at 488 nm and emission at 510 nm. The optical density was measured at 600 nm. Different induction concentration of IPTG was used to stimulate the expression. Experiments where conducted in an infinite M1000 PRO plate reader. Graphs show the mean values of fluorescents per bacteria of 6 biological replicates. The error bars represent the 95 % confidence interval. Results seem to indicate that 10 mM IPTG will give the most effective response, that the IPTG promoter is very sensitive. We make that statement from the fact that our smallest induction concentration(0,025mM) induced almost the same amount of fluorescence as our next strongest induction concentration (1mM). | ||
| − | [[File:T--Linkoping_Sweden--iptginductionparts.png|thumb|600px|center| The figure shows IPTG stimulation of the biobrick BBa_I746909 over 16 hours, the mesurments was carried out in a 96 well plate reader at 37 | + | [[File:T--Linkoping_Sweden--iptginductionparts.png|thumb|600px|center| The figure shows IPTG stimulation of the biobrick BBa_I746909 over 16 hours, the mesurments was carried out in a 96 well plate reader at 37 ͦC.The induction was carried out in a M1000 Pro plate reader. Fluorescence was exited at 488 nm and emission was measured at 510 nm . Light scatter was exited at 600 nm.]] |
| − | [[File:T--Linkoping_Sweden--stapeliptg.png|thumb|600px|center| The figure shows the end results after a 16h IPTG induction of BBa_I746909 at 37 | + | [[File:T--Linkoping_Sweden--stapeliptg.png|thumb|600px|center| The figure shows the end results after a 16h IPTG induction of BBa_I746909 at 37 ͦC. The induction was carried out in a M1000 Pro plate reader. Fluorescence was exited at 488 nm and emission was measured at 510 nm . Light scatter was exited at 600 nm. The error bars represents a 95% confidence interval.]] |
Revision as of 12:08, 17 October 2018
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_I746909
2018 iGEM team Linkoping Sweden
2018 iGEM team Linkoping Sweden validated this part.
[http://2014.igem.org/Team:Imperial iGEM Team Imperial
[http://2014.igem.org/Team:Aachen iGEM Team Aachen
The iGEM Team Aachen used the biobrick I746909 to test their [http://2014.igem.org/Team:Aachen/Project/2D_Biosensor sensor chip technology].

|
| Testing I746909 in sensor chips I746909 in sensor chips induced with 2µl IPTG and measured with a plate reader. Top chip is not induced, bottom chip is induced with IPTG. |
The biorbick I746909 was also used to test the measurement device [http://2014.igem.org/Team:Aachen/Project/Measurement_Device WatsOn].

|
| Testing I746909 in WatsOn I746909 in sensor chips induced with 2µl IPTG and measured with WatsOn. Left chip is not induced, right chip is induced with IPTG. |
2015 iGEM team Bielefeld-CeBiTec
2015 iGEM team Bielefeld-CeBiTec used this part and improved it by addition of a designed, translation enhancing 5'-untranslated region (5'-UTR; BBa_K1758100). You can find further information at BBa_K1758102).
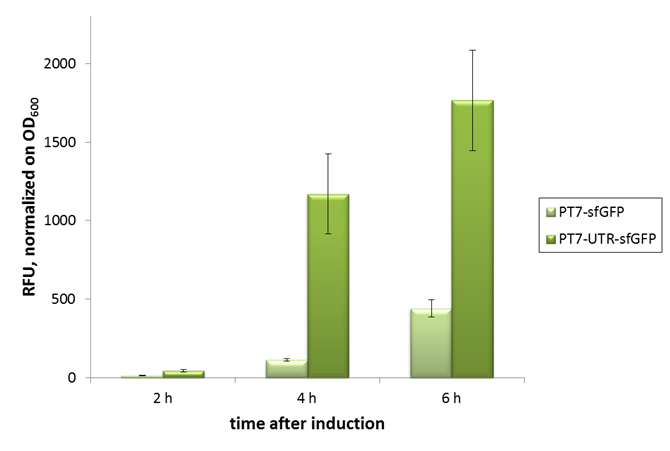
As can be seen in the picture, the difference was observable with the naked eye as well.
[http://2014.igem.org/Team:Imperial iGEM Team Imperial
[http://2014.igem.org/Team:Aachen iGEM Team Aachen
The iGEM Team Aachen used the biobrick I746909 to test their [http://2014.igem.org/Team:Aachen/Project/2D_Biosensor sensor chip technology].

|
| Testing I746909 in sensor chips I746909 in sensor chips induced with 2µl IPTG and measured with a plate reader. Top chip is not induced, bottom chip is induced with IPTG. |
The biorbick I746909 was also used to test the measurement device [http://2014.igem.org/Team:Aachen/Project/Measurement_Device WatsOn].

|
| Testing I746909 in WatsOn I746909 in sensor chips induced with 2µl IPTG and measured with WatsOn. Left chip is not induced, right chip is induced with IPTG. |
User Reviews
UNIQ5f6bbc0b34099aa5-partinfo-00000006-QINU UNIQ5f6bbc0b34099aa5-partinfo-00000007-QINU



