Difference between revisions of "Part:BBa K2586003"
Dschannek96 (Talk | contribs) |
(→Purification and ICTS with aroA) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2586003 short</partinfo> | <partinfo>BBa_K2586003 short</partinfo> | ||
| − | + | <br> | |
| − | This part encodes for the 3-phosphoshikimate 1-carboxyvinyltransferase. <br/> | + | <html> |
| + | <p align="justify"> | ||
| + | This part encodes for the 3-phosphoshikimate 1-carboxyvinyltransferase. <br/><br> | ||
The encoded enzyme is needed for the biosynthesis of essential aromatic amino acids in the shikimate pathway. It is essential in <i>Bacillus subtilis </i>, as three essential aromatic amino acids are products of the pathway: Trp, Phe and Tyr. <br/> | The encoded enzyme is needed for the biosynthesis of essential aromatic amino acids in the shikimate pathway. It is essential in <i>Bacillus subtilis </i>, as three essential aromatic amino acids are products of the pathway: Trp, Phe and Tyr. <br/> | ||
<i>aroE</i> encodes the 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase, which is the main target of the herbicide glyphosate and is strongly inhibited through glyphosate application. | <i>aroE</i> encodes the 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase, which is the main target of the herbicide glyphosate and is strongly inhibited through glyphosate application. | ||
| − | An amplification of <i>aroE</i> allows for glyphosate resistance | + | An amplification of <i>aroE</i> allows for glyphosate resistance, this is used in multiple GM plants to increase the yield and improve farming applications. |
| + | </p> | ||
| + | </html> | ||
| + | [[File:T--goettingen--BBa K2586007 structureAroE.jpg|300px|right|thumb|'''Fig. 1.'''<b>Bildunterschrift</b>]] | ||
| + | <html> | ||
| + | <br> | ||
| + | <style type="text/css"> | ||
| + | .tg {border-collapse:collapse;border-spacing:0;} | ||
| + | .tg td{font-family:Arial, sans-serif;font-size:14px;padding:0px 20px;border-style:solid;border-width:0px;overflow:hidden;word-break:normal;border-top-width:1px;border-bottom-width:1px;border-color:black;} | ||
| + | .tg th{font-family:Arial, sans-serif;font-size:14px;font-weight:normal;padding:0px 20px;border-style:solid;border-width:0px;overflow:hidden;word-break:normal;border-top-width:1px;border-bottom-width:1px;border-color:black;} | ||
| + | .tg .tg-4ovm{font-weight:bold;font-size:13px;border-color:#000000;text-align:left;vertical-align:top} | ||
| + | .tg .tg-9mbj{font-size:13px;border-color:#000000;text-align:left;vertical-align:top} | ||
| + | </style> | ||
| + | <table class="tg"> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Locus</td> | ||
| + | <td class="tg-9mbj">BSU22600</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Isoelectric point</td> | ||
| + | <td class="tg-9mbj">6.34<br></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Molecular weight</td> | ||
| + | <td class="tg-9mbj">45.08 kDa</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Protein length</td> | ||
| + | <td class="tg-9mbj">428 aa</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Gene length</td> | ||
| + | <td class="tg-9mbj">1284 bp<br></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Function</td> | ||
| + | <td class="tg-9mbj">biosynthesis of aromatic amino acids</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Product</td> | ||
| + | <td class="tg-9mbj">3-phosphoshikimate 1-carboxyvinyltransferase<br></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Essential</td> | ||
| + | <td class="tg-9mbj">yes</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </html> | ||
| + | |||
| + | <p> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| Line 14: | Line 65: | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K2586003 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2586003 SequenceAndFeatures</partinfo> | ||
| − | |||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
| Line 20: | Line 70: | ||
<partinfo>BBa_K2586003 parameters</partinfo> | <partinfo>BBa_K2586003 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | |||
| + | ==Characterization== | ||
| + | ===<i>aroE</i> is essential in <i>B. subtilis </i>=== | ||
| + | |||
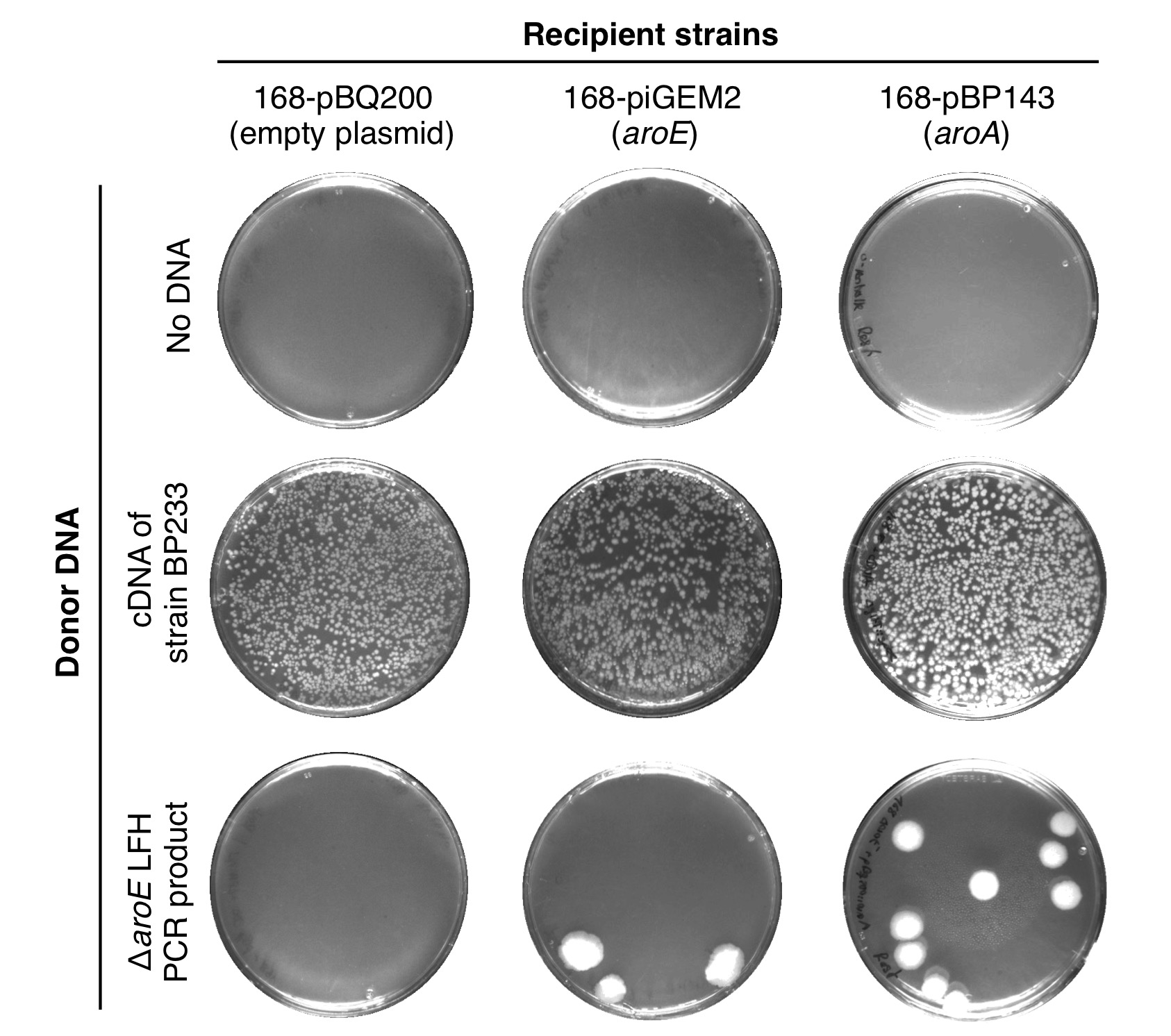
| + | [[File:T--goettingen--aroE essentiality.jpg|400px|thumb|'''Fig. 1.''' <b>The EPSP synthase is essential in <i>B. subtilis.</i></b> Transformation experiment to evaluate the essentiality of the EPSP synthase in B. subtilis. The bacteria were propagated on SP plates supplemented with spectinomycin and incubated for 24 h at 37°C.]] | ||
| + | |||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | |||
| + | As essential bacterial genes appear to be more conserved than nonessential genes, the aroE gene probably does not permit the accumulation of mutations, which can be beneficial (Jordan et al., 2002). To confirm that the aroE gene is indeed essential, we transformed the B. subtilis with a PCR product consisting of DNA fragments flanking the target gene and the intervening spc spectinomycin gene (aroE::spc deletion cassette) and propagated the bacteria on SP rich medium plates (see Notebook). Several tiny colonies appeared on the transformation plates after 24 h of incubation. However, the potential transformants were not viable and supplementation of the agar plates with casamino acids also did not improve growth of the bacteria. Thus, under the tested conditions aroE seems to be essential in B. subtilis. Next, we tested whether the chromosomal copy of the aroE gene becomes dispensable for the bacteria carrying an EPSP synthase gene on a plasmid. For this purpose, we transformed the B. subtilis wild type strain 168 with the plasmids pIGEM2 and pBP143 containing the aroE and aroA EPSP synthase genes from B. subtilis and E. coli, respectively. The wild type carrying the empty plasmid pBQ200 served as a control. Next, we transformed the three strains with the aroE::spc deletion cassette and with chromosomal DNA of strain BP233 (gltT::spc), of which the latter served as the positive control. While we did not get transformants without DNA, many transformants appeared with chromosomal DNA of strain BP233 (Figure X). Moreover, the chromosomal copy of the aroE gene was only dispensable in strains carrying an extra EPSP synthase gene on a plasmid. To conclude, the aroE EPSP synthase gene is essential in B. subtilis. Moreover, the bacteria seem to require the enzymatic function of the EPSP synthase because also the enzyme from E. coli permits growth of the aroE mutant | ||
| + | </p> | ||
| + | <br style="clear: both" /> | ||
| + | </html> | ||
| + | <hr> | ||
| + | ===Purification and ICTS with AroE=== | ||
| + | |||
| + | [[File:T--goettingen--aroE_aroA_Strep_ITC_Marburg.jpg|400px|thumb|'''Fig. 2.''' <b>(A)</b> Evaluation of the purification of the N-terminally Strep-tagged EPSP synthases from B. subtilis and E. coli Strep-AroE and Strep-AroA, respectively, by 12% SDS PAGE. The proteins were stained with Coomassie Brilliant Blue. M, unstained protein molecular weight marker Thermo Scientific; CE, crude extract; FT, flow through, W, washing steps; E, elution steps. (B) ITC measurements with Strep-AroE and Strep-AroA and glyphosate. (C) Control experiments with water and the calcium chelator EDTA.]] | ||
| + | |||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | |||
| + | We investigated together with the iGEM Team Marburg the interaction of the EPSP synthases AroE and AroA from <i>B. subtilis</i> and <i>E. coli</i>, respectively, with glyphosate. Glyphosate targets AroE and AroA in <i>B. subtilis</i> and <i>E. coli</i>, respectively (see Background information). Since the members of the Bange lab, which is hosting the iGEM team of Marburg, are experts in the field of Isothermal titration calorimetry (ITC), we did not hesitate to contact them for support. Our team cloned the aroE and aroA genes <i>B. subtilis</i> and <i>E. coli</i>, respectively, and the resulting constructs are suitable for overexpression of the Strep-tagged AroE and AroA enzymes in the E. coli strain BL21. The N-terminally Strep-tagged proteins can be purified by Streptactin:Strep-tag affinity purification system from the IBA, Göttingen. The purified proteins were send to the iGEM team in Marburg (Figure X). The ITC measurments were performed in Marburg. Unfortunately, not interaction between glyphosate and the EPSP synthases could be detected. The experiments will be repeated using freshly purified proteins. | ||
| + | |||
| + | </p> | ||
| + | <br style="clear: both" /> | ||
| + | </html> | ||
Latest revision as of 16:45, 24 September 2018
aroE: 3-phosphoshikimate 1-carboxyvinyltransferase
This part encodes for the 3-phosphoshikimate 1-carboxyvinyltransferase.
The encoded enzyme is needed for the biosynthesis of essential aromatic amino acids in the shikimate pathway. It is essential in Bacillus subtilis , as three essential aromatic amino acids are products of the pathway: Trp, Phe and Tyr.
aroE encodes the 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase, which is the main target of the herbicide glyphosate and is strongly inhibited through glyphosate application.
An amplification of aroE allows for glyphosate resistance, this is used in multiple GM plants to increase the yield and improve farming applications.
| Locus | BSU22600 |
| Isoelectric point | 6.34 |
| Molecular weight | 45.08 kDa |
| Protein length | 428 aa |
| Gene length | 1284 bp |
| Function | biosynthesis of aromatic amino acids |
| Product | 3-phosphoshikimate 1-carboxyvinyltransferase |
| Essential | yes |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 90
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 415
- 1000COMPATIBLE WITH RFC[1000]
Characterization
aroE is essential in B. subtilis
As essential bacterial genes appear to be more conserved than nonessential genes, the aroE gene probably does not permit the accumulation of mutations, which can be beneficial (Jordan et al., 2002). To confirm that the aroE gene is indeed essential, we transformed the B. subtilis with a PCR product consisting of DNA fragments flanking the target gene and the intervening spc spectinomycin gene (aroE::spc deletion cassette) and propagated the bacteria on SP rich medium plates (see Notebook). Several tiny colonies appeared on the transformation plates after 24 h of incubation. However, the potential transformants were not viable and supplementation of the agar plates with casamino acids also did not improve growth of the bacteria. Thus, under the tested conditions aroE seems to be essential in B. subtilis. Next, we tested whether the chromosomal copy of the aroE gene becomes dispensable for the bacteria carrying an EPSP synthase gene on a plasmid. For this purpose, we transformed the B. subtilis wild type strain 168 with the plasmids pIGEM2 and pBP143 containing the aroE and aroA EPSP synthase genes from B. subtilis and E. coli, respectively. The wild type carrying the empty plasmid pBQ200 served as a control. Next, we transformed the three strains with the aroE::spc deletion cassette and with chromosomal DNA of strain BP233 (gltT::spc), of which the latter served as the positive control. While we did not get transformants without DNA, many transformants appeared with chromosomal DNA of strain BP233 (Figure X). Moreover, the chromosomal copy of the aroE gene was only dispensable in strains carrying an extra EPSP synthase gene on a plasmid. To conclude, the aroE EPSP synthase gene is essential in B. subtilis. Moreover, the bacteria seem to require the enzymatic function of the EPSP synthase because also the enzyme from E. coli permits growth of the aroE mutant
Purification and ICTS with AroE
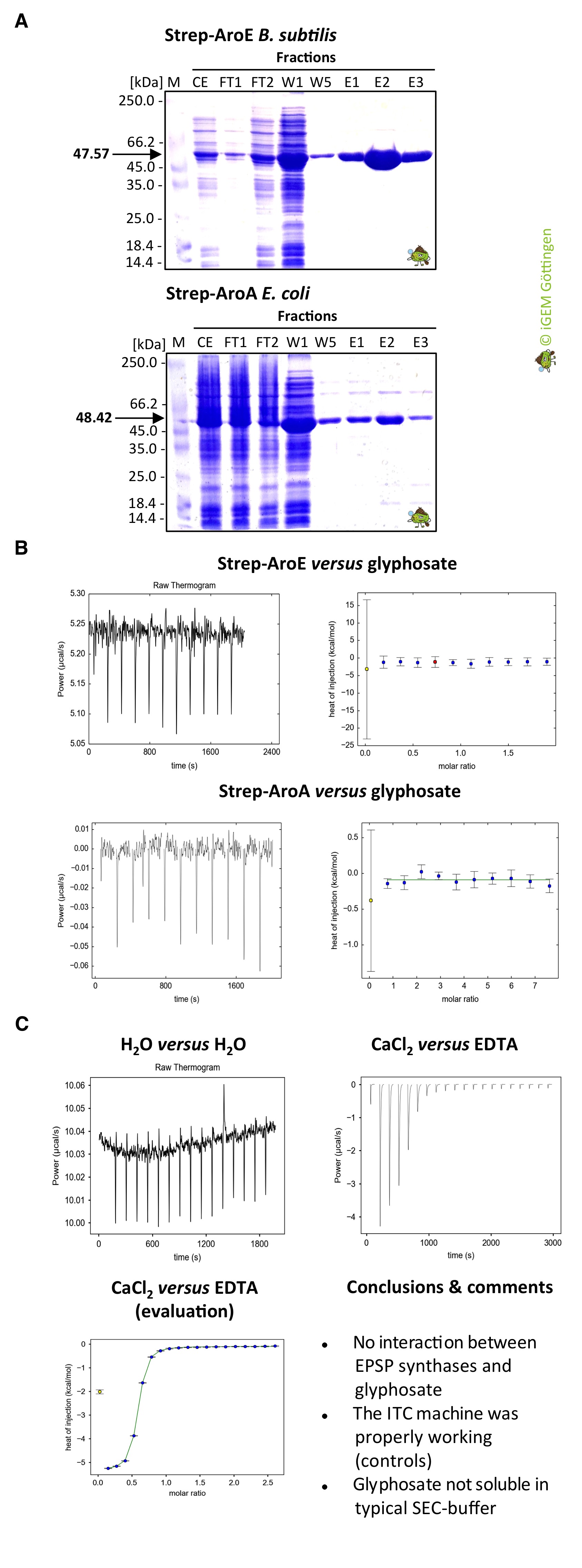
We investigated together with the iGEM Team Marburg the interaction of the EPSP synthases AroE and AroA from B. subtilis and E. coli, respectively, with glyphosate. Glyphosate targets AroE and AroA in B. subtilis and E. coli, respectively (see Background information). Since the members of the Bange lab, which is hosting the iGEM team of Marburg, are experts in the field of Isothermal titration calorimetry (ITC), we did not hesitate to contact them for support. Our team cloned the aroE and aroA genes B. subtilis and E. coli, respectively, and the resulting constructs are suitable for overexpression of the Strep-tagged AroE and AroA enzymes in the E. coli strain BL21. The N-terminally Strep-tagged proteins can be purified by Streptactin:Strep-tag affinity purification system from the IBA, Göttingen. The purified proteins were send to the iGEM team in Marburg (Figure X). The ITC measurments were performed in Marburg. Unfortunately, not interaction between glyphosate and the EPSP synthases could be detected. The experiments will be repeated using freshly purified proteins.