Difference between revisions of "Part:BBa K2239009"
| (2 intermediate revisions by the same user not shown) | |||
| Line 78: | Line 78: | ||
The 3mL reaction solution containing 150 mM phosphate buffer(pH 8.0), 10 mM UDCA, 30 mM glucose, 0.2mM NADP+, combined with 1U/ml 7β-HSDH and 5U/ml GDH at room temperature. | The 3mL reaction solution containing 150 mM phosphate buffer(pH 8.0), 10 mM UDCA, 30 mM glucose, 0.2mM NADP+, combined with 1U/ml 7β-HSDH and 5U/ml GDH at room temperature. | ||
| − | The bioconversion experiment was monitored via HPLC measurements. The sample was analyzed by UV detection at 210nm. We testify the synthesize of 7-oxo-LCA and the decrease of UDCA, using a mobile phase of methanol–water mixture (final ratio 80:20,pH 3.5 with phosphoric acid) using C18 . | + | The bioconversion experiment was monitored via HPLC measurements. The sample was analyzed by UV detection at 210nm. We testify the synthesize of 7-oxo-LCA and the decrease of UDCA, using a mobile phase of methanol–water mixture (final ratio 80:20,pH 3.5 with phosphoric acid) using chromatographic column C18 . |
<h5>Result</h5> | <h5>Result</h5> | ||
| − | + | [[file:hplc2-1.jpeg|500px]] | |
| + | |||
| + | [Fig. 9. Absorbance of NADPH] | ||
| + | |||
| + | |||
| + | [[file:hplc2-2.jpeg|400px]] | ||
| + | |||
| + | [Fig. 10. Peak area of 7oxo-LCA sample in different concentration] | ||
| + | |||
| + | |||
| + | [[file:hplc2-3.jpeg|500px]] | ||
| + | |||
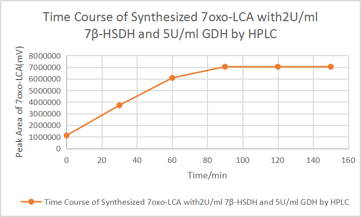
| + | [Fig. 11. Time course] | ||
| + | |||
| + | |||
| + | According to the HPLC result after 90minute, there is no significant increase of 7oxo-LCA, as a result, most of the UDCA has been transformed into 7oxo-LCA. And according to the HPLC the final yielding rate is 93%. | ||
| + | |||
| + | According to the Absorbance of NADPH that shown in figure, the absorbance is decreased significantly after 90 minute dual to the depleted UDCA that stop the conversion of UDCA to 7oxo-LCA and NADP+ synthesize. Because of the abundant amount of glucose in the solution, the GDH that works on glucose still regenerate the NADPH by taking a pair of hydrogen from glucose until most of the NADP+ transformed into NADPH. | ||
Latest revision as of 17:50, 1 November 2017
CBD-GDH
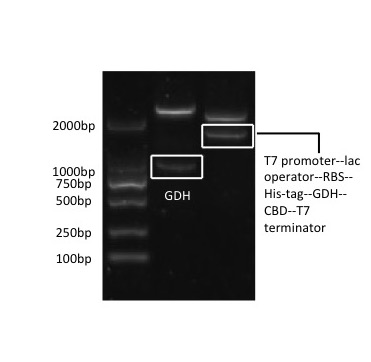
CBD-GDH (T7 promoter--lac operator--RBS--His-tag--GDH--CBD--T7 terminator)
This device codes for the GDH-CBD fusion protein.
Construct
The vector of GDH-CBD for its expression is pET-28x. It is formed by modifying the restriction enzyme sites EcoR I and Xba I of vector pET-28a.
The GDH sequence is cloned from the genome of Bacillus subtilis through PCR amplification, using the primers designed and synthesized based on its sequence. The restriction site BamH I is added to the upstream primer, and Hind III is added to the downstream primer.
The CBD sequence is retrieved from the GenBank. It is artificially synthesized and inserted into plasmid pUC57. The CBD gene is then cloned from the plasmid by PCR amplification, with the restriction site Hind III added to the upstream primer, and Xhol I added to the downstream primer.
Firstly, the GDH gene is inserted into the modified pET-28x at BamH I and Hind III, and CBD at Hind III and Xhol I, after proliferation in T3 vector. Then the whole gene fragment, T7 promoter--lac operator--RBS--His-tag--GDH--CBD--T7 terminator, is retrieved from this plasmid by PCR amplification, with prefix containing EcoR I, Not I and Xba I added on its upstream primer, and suffix containing Pst I, Not I and Spe I added on its downstream primer. The PCR product is then connected to pSB1C3 at EcoR I and Pst I.
[Fig. 1. pSB1C3-CBD-GDH]
Usage and Biology
GDH(glucose dehydrogenase) catalyzes the conversion of beta-D-glucose to D-glucono-1,5-lactone and back, as it converts NADP+ to NADPH and back. It is used to catalyze the oxidation of beta-D-glucose into D-glucono-1,5-lactone and reduce NADP+ into NADPH during the process.[1]
CBD (cellulose binding domain) is able to bind to cellulose. When connected to GDH, CBD is able to immobilize the enzyme GDH after expression, by binding to the gauze inside the solution on its cellulose.[2]
The function of cellulose binding domain
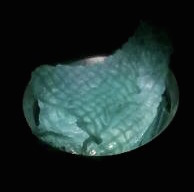
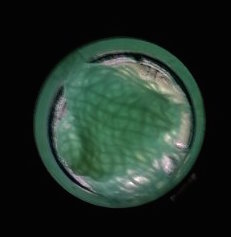
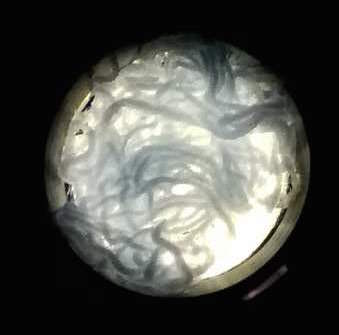
The function of CBD is tested by connecting CBD gene with GFP gene in pET28x. The GFP-CBD fusion protein is expressed and mixed with a gauze piece. The green fluorescent on the gauze is not significantly reduced after washing, proving that the CDB is well functioned. In comparison, no green fluorescent is left after washing the gauze mixed with GFP-ChBD (Chintin binding domain).
[Fig. 2. GFP-CBD on gauze before washing]
[Fig. 3. GFP-CBD on gauze after washing]
[Fig. 4. GFP-ChBD on gauze before washing]
[Fig. 5. GFP-ChBD on gauze after washing]
Expression and Immobilization[2]
The constructed pET28x-GDH plasmid is transformed into BL21(DE3) E.coli for expression. After that, when the OD 600 reached 0.6-0.8, 0.2mM IPTG is added in the liquid culture. The mixture is shaken at 20 ℃ overnight. The bacteria is collected by centrifugation at low temperature, 8000 rpm for 10 minutes, and the supernatant is discarded. The bacteria is then resuspended using 0.15M pH8.8 Tris-HCL, and is broken by ultrasonication.
The resulted bacteria solution is diluted to a certain concentration and mixed with gauze piece and the gauze piece is washed three times by ddH2O afterwards. As a result, the CBD protein binds to the cellulose on gauze, and the enzyme is successfully immobilized.
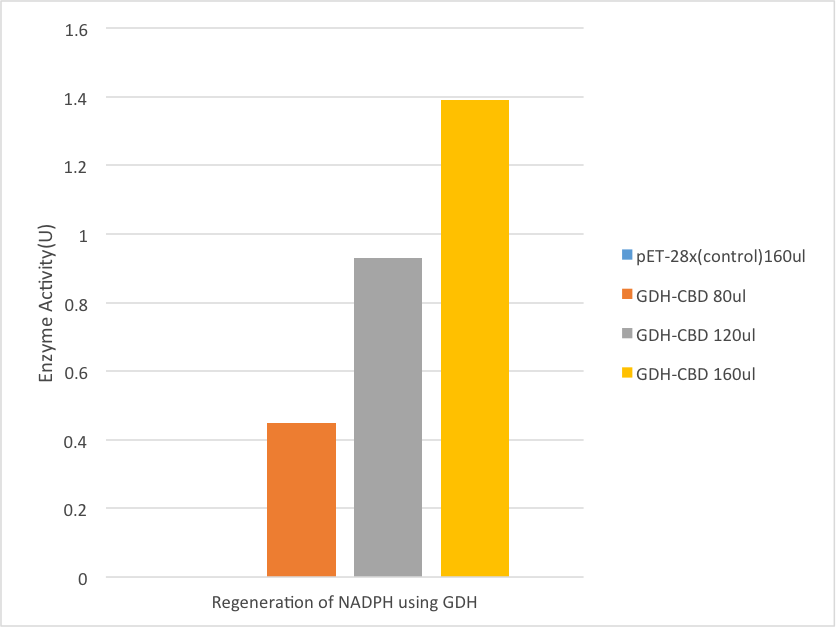
Enzyme Activity
Regeneration of NADPH
GDH is an NADPH dependent enzyme from bacillus subtilis. The 3mL reaction consists of 150mM phosphate buffer(pH 6.5), 30mM glucose, 0.2mM NADP+. The reaction started when the solution is combined with GDH-CBD-enzyme-binding gauze that in different concentration, includes 80ul, 120ul, and 160ul liquid supernatant of ultrasonication bacteria solution. The control group was testify under the same solution and condition but using pET28x-CBD liquid supernatant of ultrasonication bacteria solution to bind with gauze in the concentration of 160ul. Before adding the gauze into the solution, the gauze was washed by ddH2O for 3 times in order to purify the enzyme.
The GDH works on glucose and take a pair of hydrogen(2H+and 2e-) from glucose and add to NADP+. The NADPH was regenerate through this reaction. Since the NADPH can be testified under 340nm of ultraviolet light, the enzyme activity was determined spectrophotometrically atv340 nm (ε = 6.22 mM-1 cm-1) and room temperature by measuring the synthesize of N. One unit of activity is defined as the amount of enzyme catalyzing the synthesize of 1 mmol of NADH per min under the assay conditions used.
Result
[Fig. 6. Result]
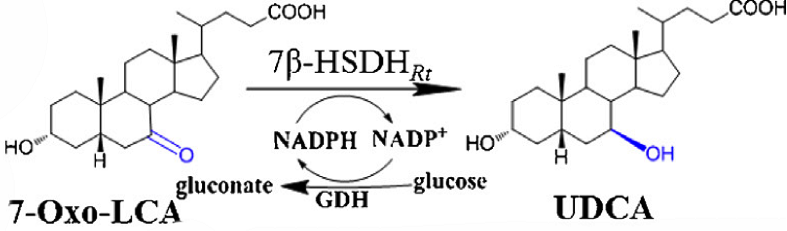
Reduction of 7-oxo-LCA to UDCA using 7β-HSDH and GDH(NADPH regeneration)
[Fig. 7. Reaction process]
The 3mL reaction solution containing 150 mM phosphate buffer(pH 8.0), 10 mM UDCA, 30 mM glucose, 0.2mM NADP+, combined with 1U/ml 7β-HSDH and 5U/ml GDH at room temperature.
The bioconversion experiment was monitored via HPLC measurements. The sample was analyzed by UV detection at 210nm. We testify the synthesize of 7-oxo-LCA and the decrease of UDCA, using a mobile phase of methanol–water mixture (final ratio 80:20,pH 3.5 with phosphoric acid) using chromatographic column C18 .
Result
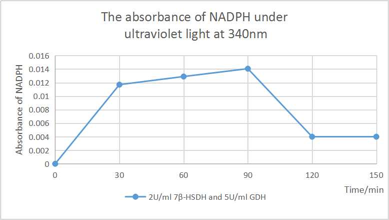
[Fig. 9. Absorbance of NADPH]
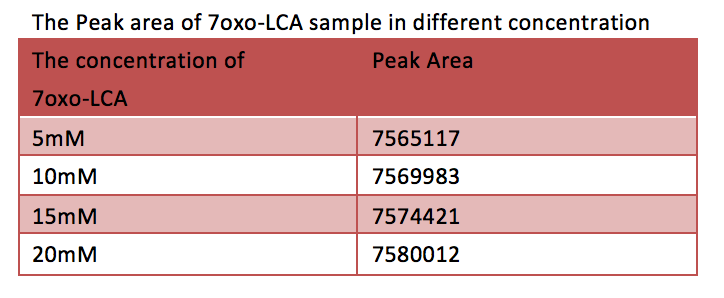
[Fig. 10. Peak area of 7oxo-LCA sample in different concentration]
[Fig. 11. Time course]
According to the HPLC result after 90minute, there is no significant increase of 7oxo-LCA, as a result, most of the UDCA has been transformed into 7oxo-LCA. And according to the HPLC the final yielding rate is 93%.
According to the Absorbance of NADPH that shown in figure, the absorbance is decreased significantly after 90 minute dual to the depleted UDCA that stop the conversion of UDCA to 7oxo-LCA and NADP+ synthesize. Because of the abundant amount of glucose in the solution, the GDH that works on glucose still regenerate the NADPH by taking a pair of hydrogen from glucose until most of the NADP+ transformed into NADPH.
Reference
[1] Ming-Min Zheng, Ru-Feng Wang, Chun-Xiu Li, Jian-He Xu: Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques. Process Biochemistry, Elsevier, 2015.
[2] Etai Shpigel, Arie Goldlust, Gilat Efroni, Amos Avraham, Adi Eshel, Mara Dekel, Oded Shoseyov: Immobilization of Recombinant Heparinase I Fused to Cellulose-Binding Domain, 1999.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1050
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 906