Difference between revisions of "Part:BBa K1378032"
(→Team NYMU-Taipei 2017: Successfully construct suicide mechanism by T4 phage) |
|||
| Line 84: | Line 84: | ||
<b>Figure 3 </b> | <b>Figure 3 </b> | ||
| − | + | [[File:T--NYMU-Taipei--Endolysin BB PCR 2268bp.png|300px]] | |
| Line 92: | Line 92: | ||
<b>Figure 4 </b> | <b>Figure 4 </b> | ||
| − | [[File:T--NYMU-Taipei--Holin-Endolysin XmaI.png| | + | [[File:T--NYMU-Taipei--Holin-Endolysin XmaI.png|300px]] |
| Line 101: | Line 101: | ||
<b>Figure 5 </b> | <b>Figure 5 </b> | ||
| − | [[ File:T--NYMU-Taipei—Holin-Endolysin-REcheck.png| | + | [[ File:T--NYMU-Taipei—Holin-Endolysin-REcheck.png|200px]] |
Revision as of 06:01, 28 October 2017
Endolysin from lambda phage

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
The λ phage endolysin is an 18-kDa soluble protein with murein transglycosylase activity[1]. In λ lysis system, enzymatically active endolysin accumulate in cytoplasm without harm to host bacteria before 'lysis time' because the holin accumulate in CM without disturbing its integrity during this time. However, at an allele-specific time, the holin oligomerizes to form a small number of large holes, allowing the endolysin to cross the CM and attack the PG [2][3] (Fig. 2).

Characterization
We choose λ lysis system to construct suicide switch due to its high efficiency and natural occurrence, and we introduce both endolysin and holin because of their cooperativity in cell lysis, which improves the performance of our suicide switch. In our design, endolysin is controlled by a constitutive promoter while holin by inducible promoter, Plac, because high concentration of holin can cause cell death alone (Fig. 4).

We transformed the two plasmids into E.coli. Then, 10mM of inducer was applied and the growth rate was measured. Compared with the bacteria carrying blank plasmid, the efficiency of our suicide switch can be evaluated.

The Fig. 5(a) shows that the difference between the OD595nm of experimental group and control group is obvious in the late logarithmic phase. The Fig. 5(b) shows that the growth curve of the E. coli carrying blank plasmid after the addition of 10mM IPTG is nearly coincident with that without addition of IPTG, excluding the possibility that the toxicity of IPTG leads to the noticeable OD595nm’s difference. Hence, the OD595nm’s difference should be caused by the slowed growth rate or cell death, and combining the working mechanism of holin and endolysin, we believe the cell death may be the main cause and our suicide switch may have some bactericidal effect.
In this experiment, every group did not enter the stationary phase and we thought better results could be attained by prolonging culture time. One possible reason for this performance of our suicide switch is that the expression of holin and endolysin is not enough to cause cell lysis. Hence, in order to test the performance of Plac, we will construct a plasmid where the expression of GFP is under control of Plac and measure the fluorescence intensity after the addition of a gradient of IPTG. According to the results of the experiment above, we will find an appropriate concentration of IPTG to get a better result if necessary. Besides, the relatively low toxicity of holin and endolysin may be another cause and we can choose the CcdA/CcdB Type II Toxin-antitoxin system instead in our future work because it have been proved that the CcdB, a topoisomerase poison targeting the GyrA subunit of DNA gyrase, shows strong toxicity to E. coli.
References
<p>[1]Bieʼnkowska-Szewczyk, K., Lipiʼnska, B., & Taylor, A. (1981). The R gene product of bacteriophage λ is the murein transglycosylase. Molecular and General Genetics MGG, 184(1), 111-114.[2]Wang, I. N., Smith, D. L., & Young, R. (2000). Holins: the protein clocks of bacteriophage infections. Annual Reviews in Microbiology, 54(1), 799-825.
[3]Dewey, J. S., Savva, C. G., White, R. L., Vitha, S., Holzenburg, A., & Young, R. (2010). Micron-scale holes terminate the phage infection cycle. Proceedings of the National Academy of Sciences, 107(5), 2219-2223.
Team NYMU-Taipei 2017: Successfully construct suicide mechanism by T4 phage
Improvement
The gene sequences of endolysin in different species of bacteriophages are slightly different. In NYMU-Taipei 2017 team’s project, we choose the endolysin from enterobacteria phage T4. The T4 endolysin part, BBa_K112806, is released from iGEM. We successfully combine T4 endolysin with a constitutive promoter (BBa_J23106), a ribosome binding site (BBa_B0034) and a double terminator (BBa_B0010 and BBa_B0012). Furthermore, we construct T4 holin and T4 endolysin together as a functional suicide mechanism. Besides, in NYMU-Taipei 2017 team’s project, we also put the suicide mechanism into practice by constructing them with NrtA.
Result
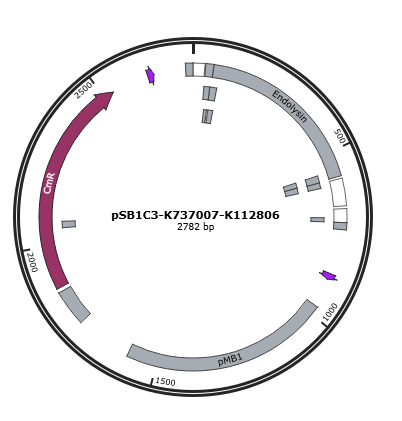
Figure 1 is the gene map of T4 endolysin construct, pSB1C3-K737007-K112806.
Figure 1
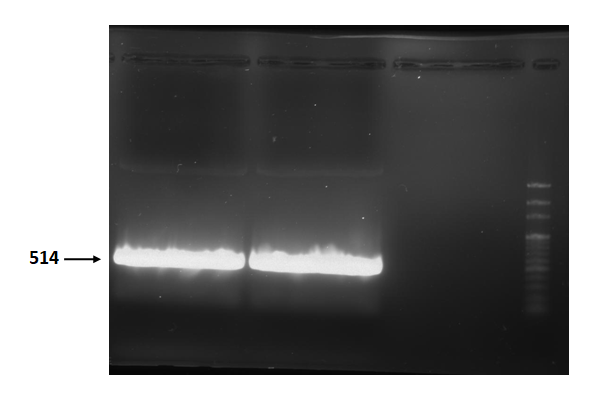
Figure 2 is electrophoresis result of endolysin (from BBa_K112806) PCR product.
The marker is 100bp. The length is 514bp as expected.
Figure 2
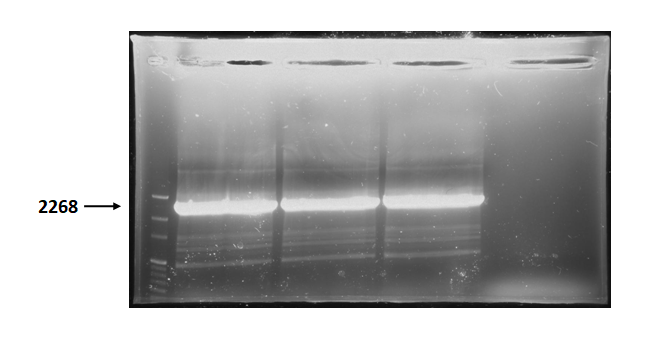
Figure 3 is electrophoresis result of endolysin backbone (from BBa_K737007) PCR product.
The marker is 100bp. The length is 2268bp as expected.
Figure 3
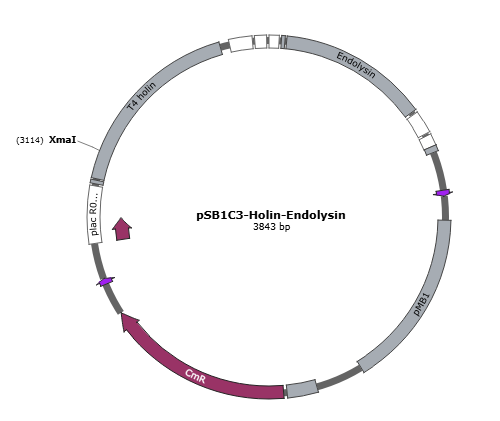
Figure 4 is the gene map of suicide mechanism construct, pSB1C3- T4 holin- T4 endolysin.
Figure 4
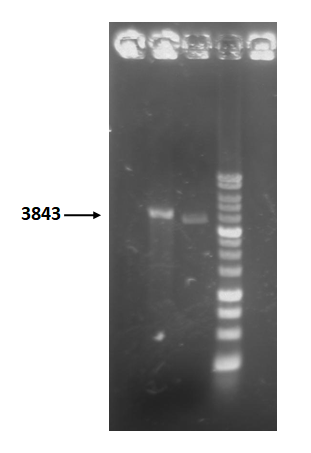
Figure 5 is restriction enzyme check electrophoresis result of T4 holin- T4 endolysin construct.
The marker is 1kb. We use XmaI to digest the sample. The total length is 3843bp as expected.
Figure 5
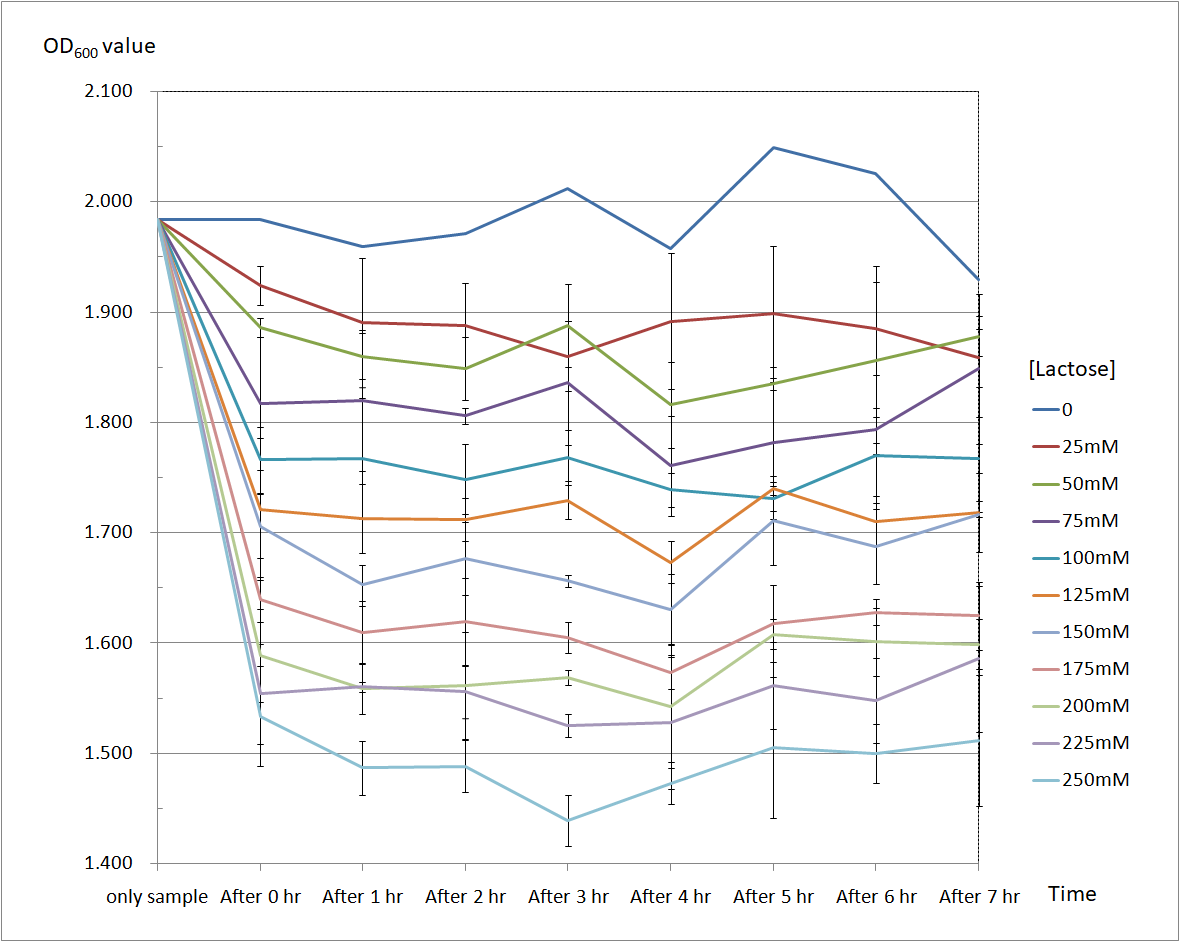
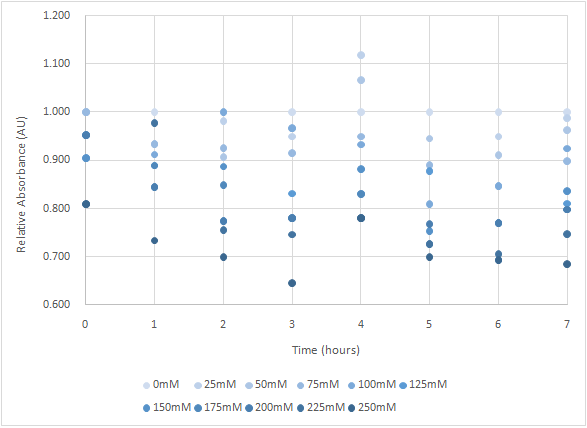
Figure 6 and Figure 7 are the results of suicide mechanism functional test. Both figure show that our suicide mechanism can be induced by adding lactose, and the effectiveness of suicide mechanism goes better as the concentration of lactose goes higher.
Figure 6 is the bacterium with T4 holin- T4 endolysin construct.
As figure 6 shows, the suicide mechanism is induced immediately when lactose is added into the samples. Besides, we can see that the lactose concentration and the OD value of the bacterium with T4 holin- T4 endolysin construct are positively correlated, which means our suicide mechanism does work.
Figure 6
Figure 7 is the bacterium with T4 holin- T4 endolysin-NrtA construct.
As figure 7 shows, the trend of the relative absorbance is downward as the lactose is added to induce the suicide mechanism. The concentration of lactose is also positively correlated with the declining degree of relative absorbance.
Figure 7