Difference between revisions of "Part:BBa K1033916:Experience"
| Line 33: | Line 33: | ||
<div align="center"> | <div align="center"> | ||
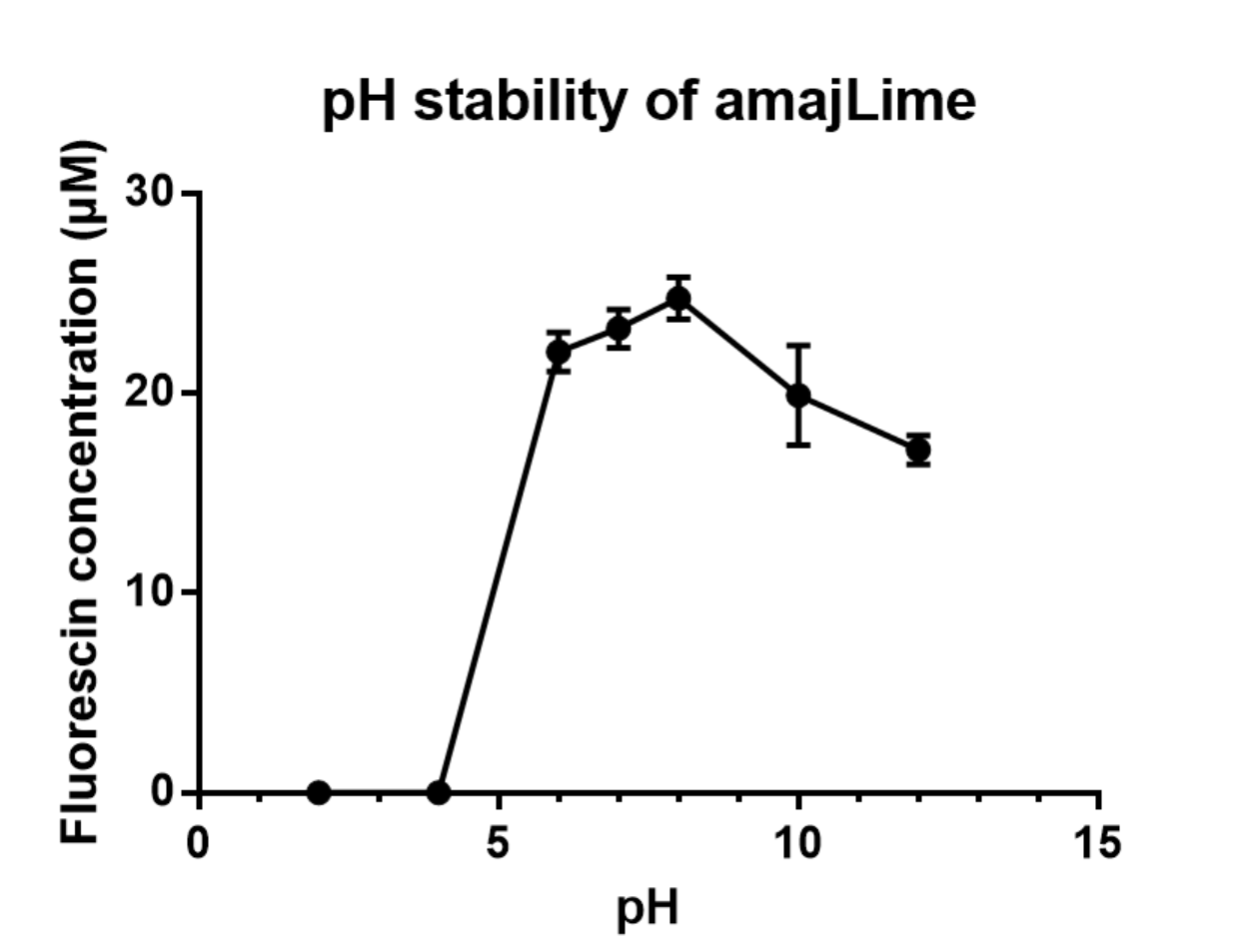
| − | [[File:Amaj.PNG |center|thumb| | + | [[File:Amaj.PNG |center|thumb|400px|''<b>Fig.2</b> Vary pH attributed to different fluorescent intensity of RFP]] |
<table cellpadding="2" border="1px" cellspacing="0" align="center" width="70%"> | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="70%"> | ||
<caption><p align="justify"><b>Table 1</b> Plate reader setting of fluorescent measurement</p></caption> | <caption><p align="justify"><b>Table 1</b> Plate reader setting of fluorescent measurement</p></caption> | ||
Revision as of 16:45, 26 August 2017
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1033916
User Reviews
UNIQ12c4a8f3f07b86e1-partinfo-00000000-QINU UNIQ12c4a8f3f07b86e1-partinfo-00000001-QINU
|
•••••
Hong Kong-CUHK iGEM 2017 |
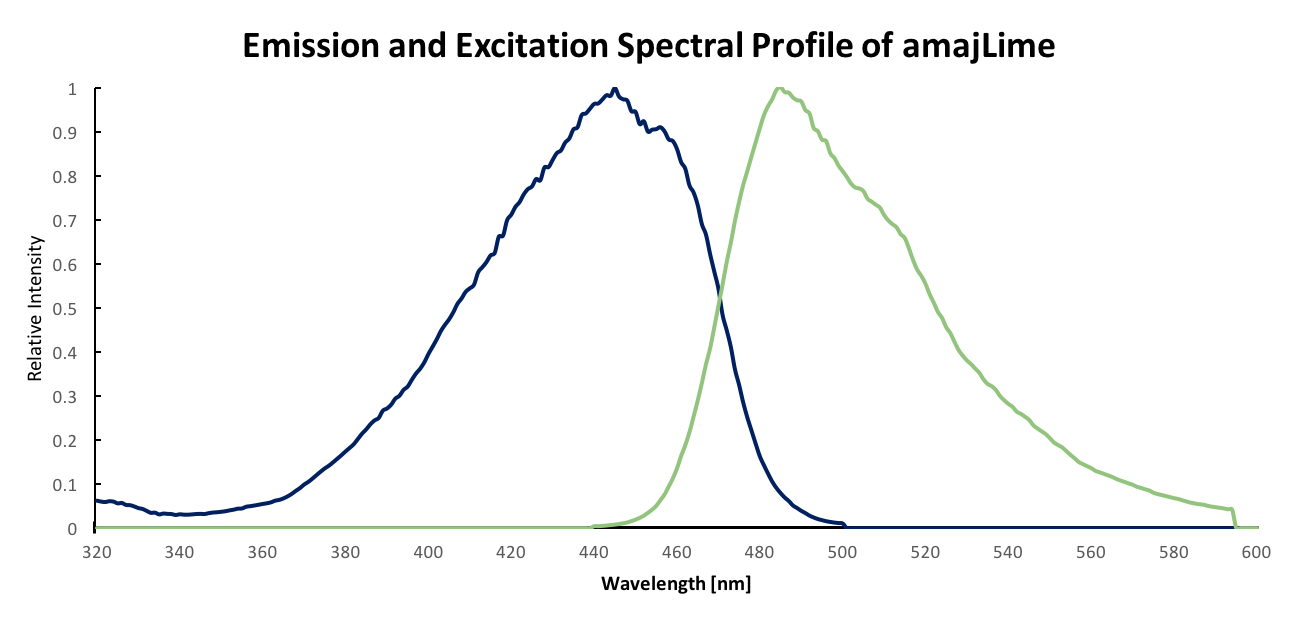
Fluorescent properties of amajLime Instead of chromorptein, we proposed that amajLime is a fluorescent protein with the property of showing pigmentation, like BBa_E1010. We charaterised spectral properties of amajLime and found its max excitation and emission wavelength at 445 nm nd 485 nm respectively.
We grew C41 bacteria with parts BBa_K1033916 with constituitive promoter: J23100, in 2XYT for 24 hours. Purifying the amajLime by Ion Exchange Chromatography and Hydrophobic Interaction Chromatography, we measured the fluoresece of purified amajLime, which is diluted to 10µg/100µl (total 200µl) in triplicates, in different buffers (ranges from pH2 to pH12). The result shows that the stability drops dramatically in condition below 6 and attains maxima fluorescence at pH 8.
|