Difference between revisions of "Part:BBa K1800000:Experience"
MonicaPolite (Talk | contribs) |
|||
| (22 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | <html> | |
| − | + | <head> | |
| − | + | <meta content="text/html; charset=ISO-8859-1" | |
| + | http-equiv="content-type"> | ||
| + | <title></title> | ||
| + | </head> | ||
| + | <body> | ||
| + | <title>BBa_K1800000</title> | ||
| + | <h2 style="margin-left: 0cm; text-indent: 0cm; font-weight: bold; font-size: 20px;">BBa_K1800000 Expression in E.coli</h2> | ||
| + | <div style="text-align:justify;"> | ||
| + | In 2015 GSU iGEM team was able to successfully express and characterize the mambalgin protein in E. coli. After inducing with IPTG overnight, the cell cultures were centrifuged and disrupted with a French press. Mambalgin was isolated using affinity chromatography, utilizing the 6x His tag in the construct. | ||
| + | <br> | ||
| + | <h2 style="margin-left: 0cm; text-indent: 0cm; text-align: center;"><a | ||
| + | name="_Toc275817880"><span lang="EN-US"></span></a><img | ||
| + | style="width: 500px; height: 500px;" alt="" | ||
| + | src="https://static.igem.org/mediawiki/parts/d/d9/Gsuigemgel.png "></h2> | ||
| + | <br> | ||
| + | <br> | ||
| + | <div style="text-align:justify;"> | ||
| + | A coomassie stain was performed to visualize the proteins. A band indicating a protein of ~9 kda – the size of the mambalgin for E. coli construct – was seen in the eluate fraction which is indicative of our desired protein. However, this band may also contain any native proteins that migrate below 11 kD (final elution contained the expected product). However the flow through and 2 washes did not have product, meaning the mambalgin protein was bound to the NiTA resin. | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <h2 style="margin-left: 0cm; text-indent: 0cm;"><a | ||
| + | name="_Toc275817880"><span lang="EN-US"></span></a></h2> | ||
| + | <h2 style="margin-left: 0cm; text-indent: 0cm; text-align: center;"><a | ||
| + | name="_Toc275817880"><span lang="EN-US"></span></a><img | ||
| + | style="width: 650px; height: 650px;" alt="" | ||
| + | src="https://static.igem.org/mediawiki/2015/d/dd/MAMBA.E.coli_Western_blot.THISONE.jpg"></h2> | ||
| + | <br> | ||
| + | <br> | ||
| + | <div style="text-align:justify;"> | ||
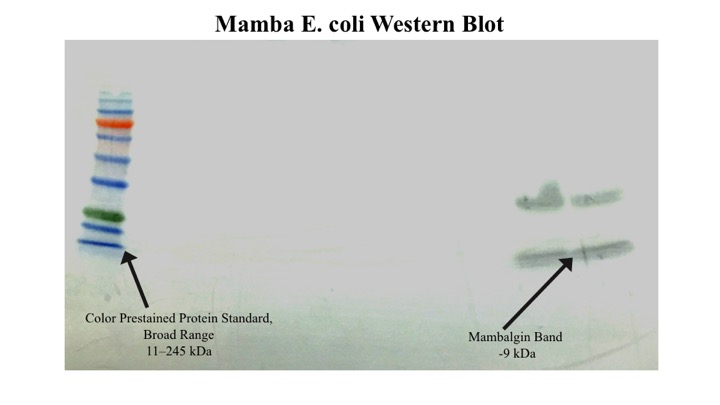
| + | In addtition, an anti-myc Western was performed to confirm the presence of mambalgin and a signal was observed at the expected size. An additional band at ~35 kd was detected however. We believe that this may be due to aggregates formed by non-specific disulfide bond formation. | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <div style="text-align:justify;"> | ||
| + | In 2016, we transformed Pichia pastoris with the mambalgin-1 gene, and continued through carrying out expression, purification, and analysis with SDS-PAGE, coomassie staining, and a Western Blot. Results of our coomassie stained gel and Western Blot are depicted below: | ||
| + | <br> | ||
<br> | <br> | ||
| − | + | <br> | |
| − | + | ||
| + | <div style="text-align:justify;"> | ||
| + | In the coomassie stained gel, a protein band showing at ~10kDa can be seen. | ||
| + | |||
| + | |||
| + | In the Western Blot, a faint band can be seen; however, because the signal was very faint, and due to constraints on heightening resolution, the picture was not uploaded. Thus we will repeat the expression in attempt to attain a higher concentration of our mambalgin-1 protein and signal intensity to confirm expression was successful. | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| − | |||
| − | |||
===Applications of BBa_K1800000=== | ===Applications of BBa_K1800000=== | ||
Latest revision as of 23:20, 29 October 2016
BBa_K1800000 Expression in E.coli
In 2015 GSU iGEM team was able to successfully express and characterize the mambalgin protein in E. coli. After inducing with IPTG overnight, the cell cultures were centrifuged and disrupted with a French press. Mambalgin was isolated using affinity chromatography, utilizing the 6x His tag in the construct.
A coomassie stain was performed to visualize the proteins. A band indicating a protein of ~9 kda – the size of the mambalgin for E. coli construct – was seen in the eluate fraction which is indicative of our desired protein. However, this band may also contain any native proteins that migrate below 11 kD (final elution contained the expected product). However the flow through and 2 washes did not have product, meaning the mambalgin protein was bound to the NiTA resin.
In addtition, an anti-myc Western was performed to confirm the presence of mambalgin and a signal was observed at the expected size. An additional band at ~35 kd was detected however. We believe that this may be due to aggregates formed by non-specific disulfide bond formation.
In 2016, we transformed Pichia pastoris with the mambalgin-1 gene, and continued through carrying out expression, purification, and analysis with SDS-PAGE, coomassie staining, and a Western Blot. Results of our coomassie stained gel and Western Blot are depicted below:
In the coomassie stained gel, a protein band showing at ~10kDa can be seen.
In the Western Blot, a faint band can be seen; however, because the signal was very faint, and due to constraints on heightening resolution, the picture was not uploaded. Thus we will repeat the expression in attempt to attain a higher concentration of our mambalgin-1 protein and signal intensity to confirm expression was successful.
===Applications of BBa_K1800000=== ===User Reviews===BBa_K1800000 StartReviews
BBa_K1800000 EndReviews
===Applications of BBa_K1800000=== ===User Reviews===