Difference between revisions of "Part:BBa C0071"
| (7 intermediate revisions by 4 users not shown) | |||
| Line 13: | Line 13: | ||
Group: <b>Tokyo Tech 2016</b> | Group: <b>Tokyo Tech 2016</b> | ||
| − | Author: Yoshio Takata | + | Author: Yoshio Takata |
| − | + | Summary of Improvement and Characterization: | |
| − | + | I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize RhlR assay | |
| + | |||
| + | II. Improvement of the wild type Prhl | ||
| + | |||
| + | III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl | ||
| + | |||
| + | |||
| + | <span style="margin-left: 10px;">We Tokyo Tech 2016 simulated our final genetic circuits and found that the circuits did not work, because Prhl strength was too weak. (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). We therefore considered using the improved Prhl (<partinfo>BBa_K1529310</partinfo>,<partinfo>BBa_K1529310</partinfo>) established by Tokyo_Tech 2014, but we noticed that they were inappropriate for two reasons (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). Then, we decided to improve Prhl Promoter and obtain our original improved Prhl (included in <partinfo>BBa_K1949060</partinfo>) that suited our goal. | ||
| + | |||
| + | <span style="margin-left: 10px;">Our purpose is to create strong Prhl for our final genetic circuits. | ||
| + | This experiment consists of the three parts below. | ||
| + | |||
| + | I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize rhlR assay | ||
| + | |||
| + | II. Improvement of the native Prhl | ||
| + | |||
| + | III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl | ||
| + | |||
| + | The past improved Prhl did not suit for our final circuits and we could create the improved Prhl appropriate to our final circuits. | ||
| + | |||
| + | |||
| + | ====I. Improved Prhl by iGEM 2014 Tokyo_Tech item and characterize RhlR assay==== | ||
<span style="margin-left: 10px;">We found that Prhl(RL) (<partinfo>BBa_K1529300</partinfo>) activity was weak and the expression level depended on LVA tag (Fig.1); LVA-tagged proteins are prone to be degraded by cellular proteases. Prhl(LR) (<partinfo>BBa_K1529310</partinfo>) activity was strong and unexpectedly reacted with C12 (crosstalk) (Fig.3). | <span style="margin-left: 10px;">We found that Prhl(RL) (<partinfo>BBa_K1529300</partinfo>) activity was weak and the expression level depended on LVA tag (Fig.1); LVA-tagged proteins are prone to be degraded by cellular proteases. Prhl(LR) (<partinfo>BBa_K1529310</partinfo>) activity was strong and unexpectedly reacted with C12 (crosstalk) (Fig.3). | ||
| Line 27: | Line 48: | ||
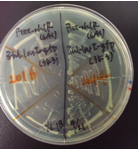
[[Image:prhl2.png|thumb|center|400px|Fig.2 The colonies of transformants with a rhlR (left) or a rhlR-LVA (right)]]<br> | [[Image:prhl2.png|thumb|center|400px|Fig.2 The colonies of transformants with a rhlR (left) or a rhlR-LVA (right)]]<br> | ||
| − | If you want more information, you see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki] and < | + | |
| + | ====II. Improvement the wild type Prhl==== | ||
| + | |||
| + | <span style="margin-left: 10px;">By introducing a single point mutation into wild type Prhl (<partinfo>BBa_R0071</partinfo>) by PCR, we obtained 198 Prhl mutants and chose the two Prhl mutants of which promoter activity was stronger than wild type Prhl(Fig.3). | ||
| + | |||
| + | [[Image:prhl4.png|thumb|center|400px|Fig. 3 RFU of GFP / turbidity of imoroved Prhl mutants]]<br> | ||
| + | |||
| + | The sequences of these two chosen mutants are shown below. The small characters indicate the scar sequence and a single point mutation is colored with red. | ||
| + | |||
| + | |||
| + | Native Prhl (<partinfo>BBa_R0071</partinfo>) | ||
| + | |||
| + | TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTAAAAAGTGTTC | ||
| + | |||
| + | Prhl(NM)(<partinfo>BBa_K1949060</partinfo>) | ||
| + | |||
| + | TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTA<font color=#ff0000><b>t</b></font>AAAGTGTTC | ||
| + | |||
| + | ====III. Comparison the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl==== | ||
| + | |||
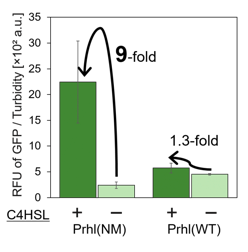
| + | <span style="margin-left: 10px;">Prhl(NM) was chosen from the many Prhl mutants, and by comparing Prhl(NM) to Prhl(LR), we obtained the result below (Fig.4). The reaction activity of Prhl(NM) to C4 was stronger than that of Prhl(LR), and Prhl(NM) did not react with C12 at all. | ||
| + | |||
| + | [[Image:prhl5.png|thumb|center|400px|Fig.4 Comparison of Prhl(NM) to Prhl(LR)]]<br> | ||
| + | |||
| + | If you want more information, you see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]! | ||
| + | |||
| + | |||
| + | ====I. Characterisation of RhlR by Imperial College London iGEM 2016 ==== | ||
| + | |||
| + | Group: <b>Imperial College London 2016</b> | ||
| + | |||
| + | We improved this part by characterising the activation range of RhlR, and the crosstalk between RhlR and non-cognate AHLs. We placed the coding sequence downstream from the pRhl promoter, and recorded the fluorescence for different concentrations of AHLs. | ||
| + | |||
| + | |||
| + | <b>Characterisation data</b> | ||
| + | |||
| + | The activation range of RhlR by its cognate inducer (C4-AHL) was characterised in TOP10 E.Coli cells. Cells transformed with the Rhl response device were cultured to the exponential phase and treated with appropriate concentrations of C4-AHL in 96-well microplates. These induced cells were grown in the microplates and their fluorescence and absorbance values (OD 600) were monitored over time using a microplate reader. The reported values for the normalised fluorescence represents the values recorded 180 minutes after AHL induction. The normalised fluorescence was calculated by dividing fluorescence values by absorbance values and correcting for LB autofluorescence. | ||
| + | |||
| + | In order to characterise the orthogonality of the Rhl system, we measured absorbance and fluorescence of the cells in the plate reader after treating them with varying concentrations of 3 different AHL signals (3O-C6 AHL of the Lux system, 3O-C12 AHL of the Las system, and 3O-C14 AHL of the Cin system). This allowed us to determine whether these non-cognate AHLs were capable of activating the RhlR response protein, and therefore the level of crosstalk between the quorum sensing systems. | ||
| + | |||
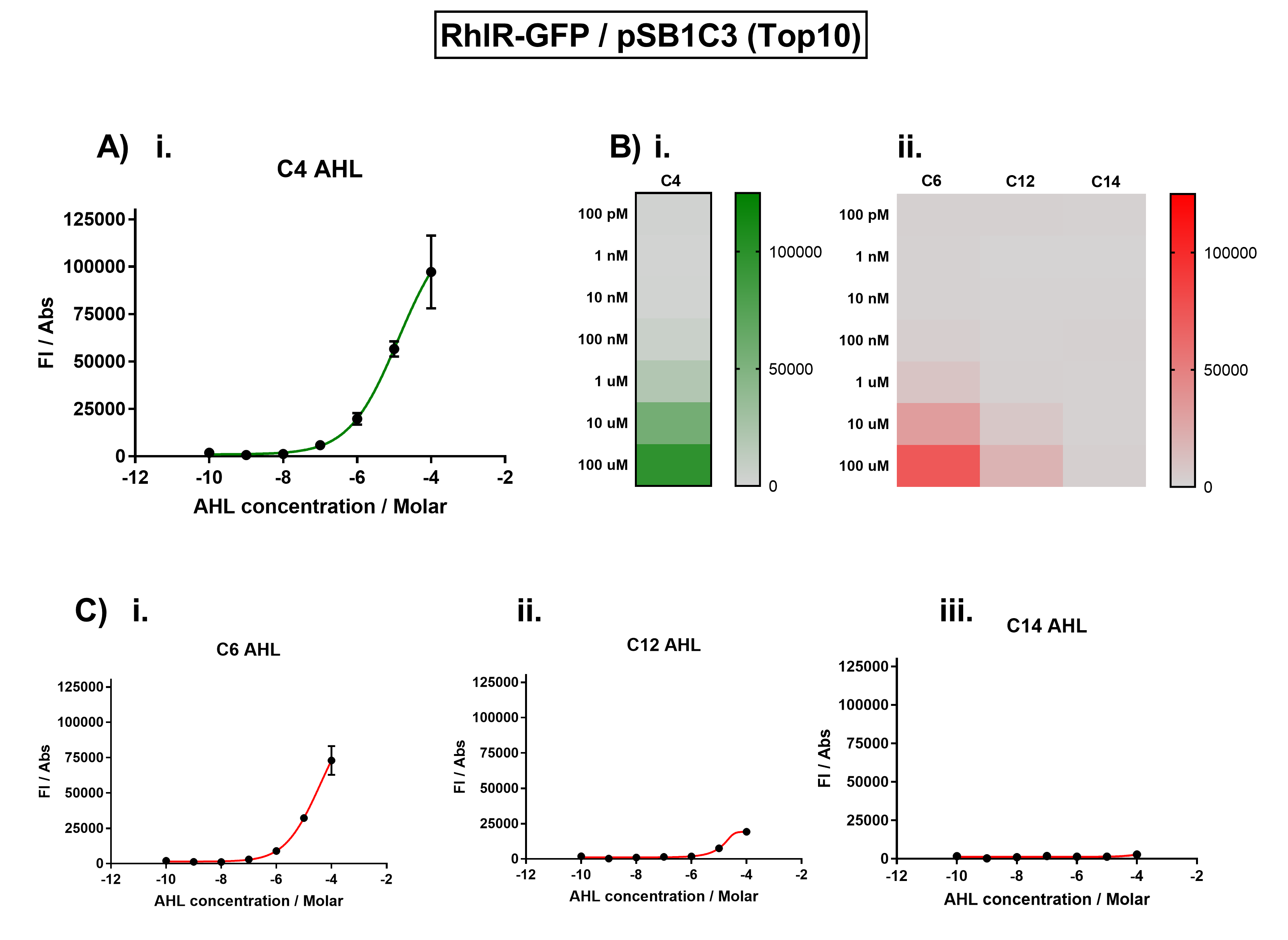
| + | [[File:IC16_BBa_K1893003_characterisation.png|700px|center|]] | ||
| + | Figure 1. Characterisation of the Rhl response device (BBa_K1893003). (A) Transfer function curve of normalised fluorescence against cognate inducer C4-AHL concentrations. (B) Heat map of normalised fluorescence of RhlR-GFP system over a range of AHL concentrations: (i) Binding of RhlR-GFP to its cognate AHL (C4 AHL). (ii) Binding of RhlR-GFP to 3 non-cognate AHLs (3O-C6 AHL, 3O-C12 AHL, 3OH-C14 AHL). (C) Transfer function curves of normalised fluorescence against non-cognate inducer AHL (C4 AHL) concentrations to investigate inducer AHL crosstalk: (i) C6-AHL (3O-C6 AHL) of the Lux system (ii) C12-AHL (3O-C12 AHL) of the Las system (iii) C14-AHL (3O-C14 AHL) of the Cin system. Experiments were performed in E. coli Top10 cell strain cultured at 37°C. Normalised fluorescence was calculated by dividing fluorescent signal by cell density (OD600). Fluorescence measurements were recorded at 180 minutes. Reported values represent the mean normalised fluorescence value from 3 technical repeats and error bars represent standard deviation of these. | ||
| + | |||
| + | <b>Analysis</b> | ||
| + | The results from Figure 1A show that the concentration range of AHL (C4 AHL) required for the activation of the Rhl response device was 100nM - 100uM. Furthermore, it can be seen from Figure 1C that RhlR does not appear to be activated by 3O-C14 AHL, suggesting that the Rhl quorum system is orthogonal with Cin. It is also seen that RhlR only exhibits slight activity at high concentrations at 3O-C12 AHL. RhlR is seen to be significantly activated by 3O-C6 AHL, suggesting that the Rhl and Lux quorum systems are not orthogonal. | ||
| + | |||
| + | |||
| + | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Latest revision as of 14:52, 29 October 2016
rhlR repressor/activator from P. aeruginosa PA3477 (+LVA)
Transcriptional regulator, in complex with N-butyryl-HSL, RhlR binds to the Rhl promoter
Usage and Biology
Transcriptional regulator, binds with N-butyryl-HSL
Characterization
Group: Tokyo Tech 2016
Author: Yoshio Takata
Summary of Improvement and Characterization:
I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize RhlR assay
II. Improvement of the wild type Prhl
III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
We Tokyo Tech 2016 simulated our final genetic circuits and found that the circuits did not work, because Prhl strength was too weak. (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). We therefore considered using the improved Prhl (BBa_K1529310,BBa_K1529310) established by Tokyo_Tech 2014, but we noticed that they were inappropriate for two reasons (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). Then, we decided to improve Prhl Promoter and obtain our original improved Prhl (included in BBa_K1949060) that suited our goal.
Our purpose is to create strong Prhl for our final genetic circuits. This experiment consists of the three parts below.
I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize rhlR assay
II. Improvement of the native Prhl
III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
The past improved Prhl did not suit for our final circuits and we could create the improved Prhl appropriate to our final circuits.
I. Improved Prhl by iGEM 2014 Tokyo_Tech item and characterize RhlR assay
We found that Prhl(RL) (BBa_K1529300) activity was weak and the expression level depended on LVA tag (Fig.1); LVA-tagged proteins are prone to be degraded by cellular proteases. Prhl(LR) (BBa_K1529310) activity was strong and unexpectedly reacted with C12 (crosstalk) (Fig.3).
The colonies of transformants with a rhlR (BBa_C0171) plasmid looked rough and the growth rate was low(Fig.2-left), while the colonies of transformants with a rhlR-LVA (BBa_C0071) plasmid looked smooth and the growth rate was normal(Fig.2-right). However, the reason for this result is unclear.
II. Improvement the wild type Prhl
By introducing a single point mutation into wild type Prhl (BBa_R0071) by PCR, we obtained 198 Prhl mutants and chose the two Prhl mutants of which promoter activity was stronger than wild type Prhl(Fig.3).
The sequences of these two chosen mutants are shown below. The small characters indicate the scar sequence and a single point mutation is colored with red.
Native Prhl (BBa_R0071)
TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTAAAAAGTGTTC
Prhl(NM)(BBa_K1949060)
TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTAtAAAGTGTTC
III. Comparison the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
Prhl(NM) was chosen from the many Prhl mutants, and by comparing Prhl(NM) to Prhl(LR), we obtained the result below (Fig.4). The reaction activity of Prhl(NM) to C4 was stronger than that of Prhl(LR), and Prhl(NM) did not react with C12 at all.
If you want more information, you see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]!
I. Characterisation of RhlR by Imperial College London iGEM 2016
Group: Imperial College London 2016
We improved this part by characterising the activation range of RhlR, and the crosstalk between RhlR and non-cognate AHLs. We placed the coding sequence downstream from the pRhl promoter, and recorded the fluorescence for different concentrations of AHLs.
Characterisation data
The activation range of RhlR by its cognate inducer (C4-AHL) was characterised in TOP10 E.Coli cells. Cells transformed with the Rhl response device were cultured to the exponential phase and treated with appropriate concentrations of C4-AHL in 96-well microplates. These induced cells were grown in the microplates and their fluorescence and absorbance values (OD 600) were monitored over time using a microplate reader. The reported values for the normalised fluorescence represents the values recorded 180 minutes after AHL induction. The normalised fluorescence was calculated by dividing fluorescence values by absorbance values and correcting for LB autofluorescence.
In order to characterise the orthogonality of the Rhl system, we measured absorbance and fluorescence of the cells in the plate reader after treating them with varying concentrations of 3 different AHL signals (3O-C6 AHL of the Lux system, 3O-C12 AHL of the Las system, and 3O-C14 AHL of the Cin system). This allowed us to determine whether these non-cognate AHLs were capable of activating the RhlR response protein, and therefore the level of crosstalk between the quorum sensing systems.
Figure 1. Characterisation of the Rhl response device (BBa_K1893003). (A) Transfer function curve of normalised fluorescence against cognate inducer C4-AHL concentrations. (B) Heat map of normalised fluorescence of RhlR-GFP system over a range of AHL concentrations: (i) Binding of RhlR-GFP to its cognate AHL (C4 AHL). (ii) Binding of RhlR-GFP to 3 non-cognate AHLs (3O-C6 AHL, 3O-C12 AHL, 3OH-C14 AHL). (C) Transfer function curves of normalised fluorescence against non-cognate inducer AHL (C4 AHL) concentrations to investigate inducer AHL crosstalk: (i) C6-AHL (3O-C6 AHL) of the Lux system (ii) C12-AHL (3O-C12 AHL) of the Las system (iii) C14-AHL (3O-C14 AHL) of the Cin system. Experiments were performed in E. coli Top10 cell strain cultured at 37°C. Normalised fluorescence was calculated by dividing fluorescent signal by cell density (OD600). Fluorescence measurements were recorded at 180 minutes. Reported values represent the mean normalised fluorescence value from 3 technical repeats and error bars represent standard deviation of these.
Analysis The results from Figure 1A show that the concentration range of AHL (C4 AHL) required for the activation of the Rhl response device was 100nM - 100uM. Furthermore, it can be seen from Figure 1C that RhlR does not appear to be activated by 3O-C14 AHL, suggesting that the Rhl quorum system is orthogonal with Cin. It is also seen that RhlR only exhibits slight activity at high concentrations at 3O-C12 AHL. RhlR is seen to be significantly activated by 3O-C6 AHL, suggesting that the Rhl and Lux quorum systems are not orthogonal.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 240
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 715