Difference between revisions of "Part:BBa K1980000"
(→References) |
|||
| (19 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K1980000 short</partinfo> | <partinfo>BBa_K1980000 short</partinfo> | ||
| − | + | ==Description== | |
| + | <p> | ||
Copper Storage protein 1 (Csp1) is a tetrameric copper storage protein found in the periplasm of Methylosinus trichosporium OB3b. We investigated whether this part could act as a copper chelator when expressed in E. coli. | Copper Storage protein 1 (Csp1) is a tetrameric copper storage protein found in the periplasm of Methylosinus trichosporium OB3b. We investigated whether this part could act as a copper chelator when expressed in E. coli. | ||
| − | We modified the protein by adding a TAT signal peptide from the E. coli enzyme CueO in place of the native TAT sequence and added a C terminal his tag to aid purification. | + | We modified the protein by adding a TAT signal peptide from the E. coli enzyme CueO in place of the native TAT sequence and added a C terminal his tag to aid purification.</p> |
<!-- --> | <!-- --> | ||
| + | <br> | ||
<span class='h3bb'>Sequence and Features:</span> | <span class='h3bb'>Sequence and Features:</span> | ||
<partinfo>BBa_K1980000 SequenceAndFeatures</partinfo> | <partinfo>BBa_K1980000 SequenceAndFeatures</partinfo> | ||
| − | + | ==Usage and Biology== | |
<p> Our project aimed to detect and chelate dietary copper as a treatment for Wilson's Disease, a copper accumulation disorder. | <p> Our project aimed to detect and chelate dietary copper as a treatment for Wilson's Disease, a copper accumulation disorder. | ||
We decided that the ideal copper chelation protein would have these properties: | We decided that the ideal copper chelation protein would have these properties: | ||
| Line 18: | Line 20: | ||
<li>As <i> E. coli </i>naturally deals with copper toxicity by binding copper in the periplasm then exporting it, periplasmic proteins may reduce toxicity to the host. </li> | <li>As <i> E. coli </i>naturally deals with copper toxicity by binding copper in the periplasm then exporting it, periplasmic proteins may reduce toxicity to the host. </li> | ||
</ul> | </ul> | ||
| − | <p>Copper storage protein 1 is a protein discovered in a methane-oxidizing alphaproteobacterium called<i>Methylosinus trichosporium OB3b. </i> (OB3b here stands for “oddball” strain 3b). This bacterium has a high demand for copper for use in its particular methane monoxygenase enzyme. Vita et al. <sup>(1)</sup> discovered Csp1 in 2015, characterised the protein’s copper affinity and obtained crystal structures with and without copper. | + | <p>Copper storage protein 1 is a protein discovered in a methane-oxidizing alphaproteobacterium called <i>Methylosinus trichosporium OB3b. </i> (OB3b here stands for “oddball” strain 3b). This bacterium has a high demand for copper for use in its particular methane monoxygenase enzyme. Vita et al.<sup>(1)</sup> discovered Csp1 in 2015, characterised the protein’s copper affinity and obtained crystal structures with and without copper. |
</p> | </p> | ||
<p> | <p> | ||
| Line 30: | Line 32: | ||
We codon optimised Csp1 to <i>E. coli</i> and replaced the original TAT sequence with a TAT sequence from the <i>E. coli</i> protein CueO, which is also involved in copper regulation. To get Csp1 from the shipping vector to the pBAD expression system for testing the TAT sequence had to be modified by the addition of a serine residue after the initiator methionine. Serine was chosen over other amino acid possibilities because other TAT sequences seemed to have serine in this location. | We codon optimised Csp1 to <i>E. coli</i> and replaced the original TAT sequence with a TAT sequence from the <i>E. coli</i> protein CueO, which is also involved in copper regulation. To get Csp1 from the shipping vector to the pBAD expression system for testing the TAT sequence had to be modified by the addition of a serine residue after the initiator methionine. Serine was chosen over other amino acid possibilities because other TAT sequences seemed to have serine in this location. | ||
</p> | </p> | ||
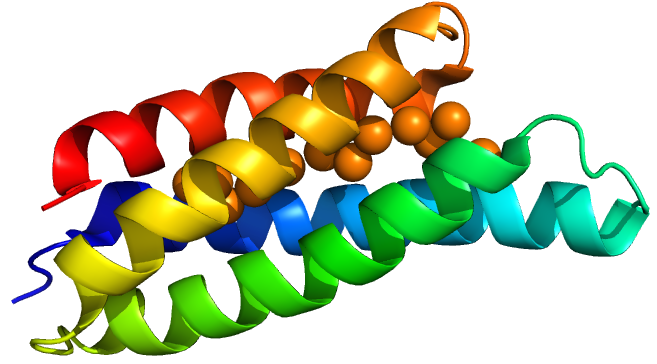
| − | + | [[Image:T--Oxford--Sam-csp1-mono.jpg|400px|thumb|centre|'''Figure 1:''' A Csp1 monomer]] | |
| − | + | ||
| − | + | ||
==Experience== | ==Experience== | ||
| − | <p>We were unable however to detect copper chelation activity of Csp1 <i> | + | <p> We cloned Csp1 from Gblock into the shipping vector then transferred it into the pBAD, arabinose-inducible commercial expression vector. This transfer necessitated the changing of the TAT sequence by the addition of a serine residue after the initiator methionine</p> |
| − | <p> | + | |
| + | ===Absorbance Assay=== | ||
| + | <html> | ||
| + | <p>We were unable however to detect copper chelation activity of Csp1 when expressed from pBAD in MG1655 <i>E. coli</i> strain, using an absorbance assay. | ||
| + | </p> | ||
| + | <p> | ||
| + | The assay used was the reagent Bathocuproine disulphonic Acid (BCS). BCS is colourless in the absence of Cu<sup>+</sup> but upon exposure to Cu<sup>+</sup>, BCS forms complexes with Cu<sup>+</sup> and absorbs strongly in the 480nm range. In our assays, at concentrations of 50µg/mL, the 480nm absorbance varied linearly with the Cu+ concentration from the detection limit of around 1µM Cu<sup>+</sup>, to approximately 20µM Cu<sup>+</sup>. As not only MymT and Csp1 bind copper in the Cu<sup>+</sup> form, but the assay also requires singly charged copper, the assays were optimised to include a suitable about of mild reducing agent to ensure reduction of the added CuSO<sub>4</sub> (releases Cu<sup>2+</sup>) to Cu<sup>+</sup>. After trying L(+)-Ascorbate and DL-Dithiothreitol, and L-Glutathione as candidates, L-Glutathione was selected as it was both mild enough to not damage biological material and efficient at reducing Cu<sup>2+</sup>. At >2-3 times the concentration of Cu<sup>2+</sup> in solution, L-Glutathione had maximum reductive activity against Cu<sup>2+</sup>.</p> | ||
| + | <p> Modelling by our team suggested that this was because insufficient protein could be expressed to chelate the amount needed to be detectable on the assay (1μM detection limit).</p> | ||
| + | <p>We purified this a version of this part with a C-terminal His tagged-sfGFP (<a href="https://parts.igem.org/Part:BBa_K1980001">BBa K198001</a>), but were still unable to detect copper chelation with the assay in our purified extracts.</p> | ||
<p> | <p> | ||
| − | When expressed from the pBAD, arabinose-inducible commercial expression plasmid, or from our copper sensitive promoter ( | + | When expressed from the pBAD, arabinose-inducible commercial expression plasmid, or from our copper sensitive promoter (<a href="https://parts.igem.org/Part:BBa_K1980010">BBa K1980010</a>) our version of Csp1sfGFP (<a href="https://parts.igem.org/Part:BBa_K1980001">BBa K1980001</a>) was observed to form inclusion bodies in the cytoplasm and not be expressed as a periplasmic protein as we had hoped:</p></html> |
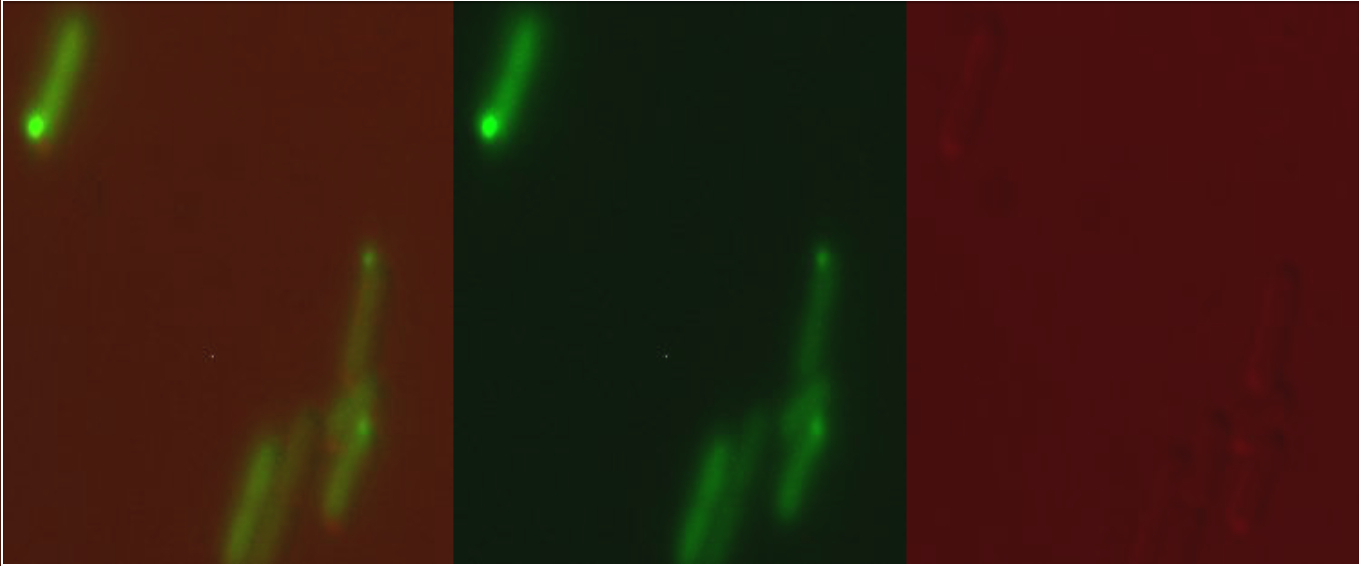
[[Image:BBa K1980000 cg microscopy.jpeg|600px|thumb|centre|'''Figure 1:''' Csp1sfGFP in the pBAD system with 2mM arabinose]] | [[Image:BBa K1980000 cg microscopy.jpeg|600px|thumb|centre|'''Figure 1:''' Csp1sfGFP in the pBAD system with 2mM arabinose]] | ||
| − | <p | + | ==References== |
| − | + | <p>(1) Nicolas Vita, Semeli Platsaki, Arnaud Basle, Stephen J. Allen, Neil G. Paterson, Andrew T. Crombie, J. Colin Murrell, Kevin J.Waldron & Christopher Dennison (2015) | |
| − | + | “A four-helix bundle stores copper for methane oxidation”, Nature 525 issue 7567 pg. 140-143 doi:10.1038/nature14854</p> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | <p style="text-align: right"><i>Authors: Sam Garforth and Andreas Hadjicharalambous</i></p> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | </p> | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | < | + | |
| − | + | ||
| − | + | ||
| − | + | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| − | <partinfo> | + | <partinfo>BBa_K1980012 parameters</partinfo> |
<!-- --> | <!-- --> | ||
Latest revision as of 21:18, 24 October 2016
TAT Copper Storage Protein 1
Description
Copper Storage protein 1 (Csp1) is a tetrameric copper storage protein found in the periplasm of Methylosinus trichosporium OB3b. We investigated whether this part could act as a copper chelator when expressed in E. coli. We modified the protein by adding a TAT signal peptide from the E. coli enzyme CueO in place of the native TAT sequence and added a C terminal his tag to aid purification.
Sequence and Features:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Our project aimed to detect and chelate dietary copper as a treatment for Wilson's Disease, a copper accumulation disorder. We decided that the ideal copper chelation protein would have these properties:
- Should be able to bind multiple copper ions per peptide to increase the efficient use of cell resources.
- They should be from the prokaryotic domain because eukaryotic proteins can have expression issues in Escherichia coli.
- As E. coli naturally deals with copper toxicity by binding copper in the periplasm then exporting it, periplasmic proteins may reduce toxicity to the host.
Copper storage protein 1 is a protein discovered in a methane-oxidizing alphaproteobacterium called Methylosinus trichosporium OB3b. (OB3b here stands for “oddball” strain 3b). This bacterium has a high demand for copper for use in its particular methane monoxygenase enzyme. Vita et al.(1) discovered Csp1 in 2015, characterised the protein’s copper affinity and obtained crystal structures with and without copper.
Csp1 is a tetramer of four-helix bundles. Each monomer can bind up to 13 Cu(I) ions meaning that the tetramer binds a maximum of 52 copper ions. Vita et al crystallised Csp1 with and without copper bound. The copper is bound inside the pre-folded helical bundles by Cys residues in contrast to metallothioneins, which are unstructured until they fold around metal ion clusters. Vita et al. found an average copper affinity of approximately 1x1017M-1.
Csp1 has a signal peptide targeting it to the twin arginine translocation pathway (TAT). This means that it is likely a periplasmic protein. However they also found cytoplasmic homologues in many species challenging their and our assumption that only copper storage occurs in the periplasm due to copper toxicity.
We codon optimised Csp1 to E. coli and replaced the original TAT sequence with a TAT sequence from the E. coli protein CueO, which is also involved in copper regulation. To get Csp1 from the shipping vector to the pBAD expression system for testing the TAT sequence had to be modified by the addition of a serine residue after the initiator methionine. Serine was chosen over other amino acid possibilities because other TAT sequences seemed to have serine in this location.
Experience
We cloned Csp1 from Gblock into the shipping vector then transferred it into the pBAD, arabinose-inducible commercial expression vector. This transfer necessitated the changing of the TAT sequence by the addition of a serine residue after the initiator methionine
Absorbance Assay
We were unable however to detect copper chelation activity of Csp1 when expressed from pBAD in MG1655 E. coli strain, using an absorbance assay.
The assay used was the reagent Bathocuproine disulphonic Acid (BCS). BCS is colourless in the absence of Cu+ but upon exposure to Cu+, BCS forms complexes with Cu+ and absorbs strongly in the 480nm range. In our assays, at concentrations of 50µg/mL, the 480nm absorbance varied linearly with the Cu+ concentration from the detection limit of around 1µM Cu+, to approximately 20µM Cu+. As not only MymT and Csp1 bind copper in the Cu+ form, but the assay also requires singly charged copper, the assays were optimised to include a suitable about of mild reducing agent to ensure reduction of the added CuSO4 (releases Cu2+) to Cu+. After trying L(+)-Ascorbate and DL-Dithiothreitol, and L-Glutathione as candidates, L-Glutathione was selected as it was both mild enough to not damage biological material and efficient at reducing Cu2+. At >2-3 times the concentration of Cu2+ in solution, L-Glutathione had maximum reductive activity against Cu2+.
Modelling by our team suggested that this was because insufficient protein could be expressed to chelate the amount needed to be detectable on the assay (1μM detection limit).
We purified this a version of this part with a C-terminal His tagged-sfGFP (BBa K198001), but were still unable to detect copper chelation with the assay in our purified extracts.
When expressed from the pBAD, arabinose-inducible commercial expression plasmid, or from our copper sensitive promoter (BBa K1980010) our version of Csp1sfGFP (BBa K1980001) was observed to form inclusion bodies in the cytoplasm and not be expressed as a periplasmic protein as we had hoped:
References
(1) Nicolas Vita, Semeli Platsaki, Arnaud Basle, Stephen J. Allen, Neil G. Paterson, Andrew T. Crombie, J. Colin Murrell, Kevin J.Waldron & Christopher Dennison (2015) “A four-helix bundle stores copper for methane oxidation”, Nature 525 issue 7567 pg. 140-143 doi:10.1038/nature14854
Authors: Sam Garforth and Andreas Hadjicharalambous