Difference between revisions of "Part:BBa K1893013"
| Line 5: | Line 5: | ||
This is the STAR construct with its own transcription terminator t500. When transcribed, it produces the STAR-antisense RNA (pAD1.A5), which can bind to the sense STAR-target attenuator sequence (pAD1.S5). Thus, only in the presence of STAR-antisense, transcription and expression of coding sequences downstream to the STAR-target is activated. | This is the STAR construct with its own transcription terminator t500. When transcribed, it produces the STAR-antisense RNA (pAD1.A5), which can bind to the sense STAR-target attenuator sequence (pAD1.S5). Thus, only in the presence of STAR-antisense, transcription and expression of coding sequences downstream to the STAR-target is activated. | ||
| − | + | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
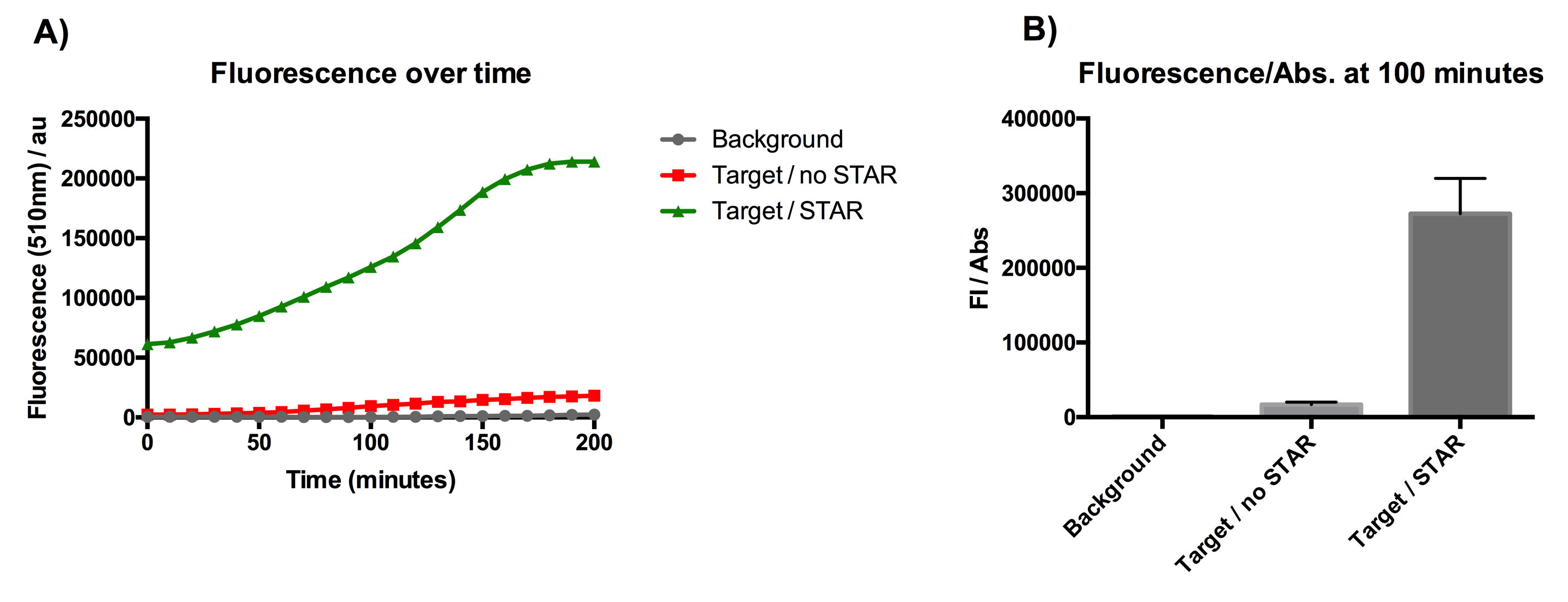
| − | + | [[File:IC16_STAR1stgraph.png|700px|center|]] | |
| + | Figure 1: Characterisation of STAR system in TOP10 E. coli cells. (A) Normalised fluorescence monitored over time for cell lines incorporating the STAR system in the absence or presence of transcribed STAR molecules (B) Normalised endpoint fluorescence (100 minutes) for cell lines in the absence or presence of STAR molecules. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. Normalised fluorescence was calculated by dividing fluorescent signal by the O.D.600 value of the culture. Background was determined by the use of DH10B cells with no plasmid transformed. Error bars represent standard deviation from 3 technical repeats. | ||
| + | |||
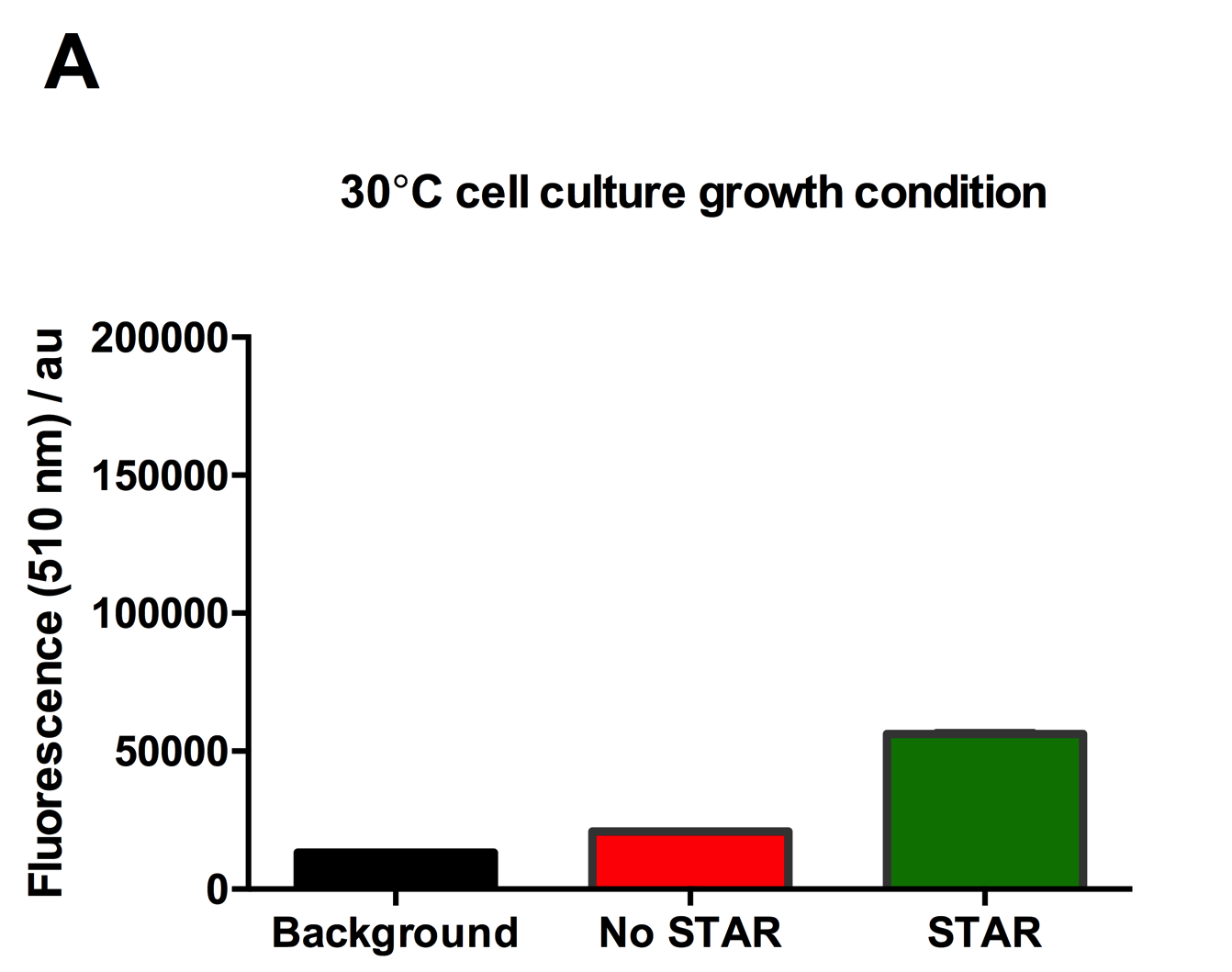
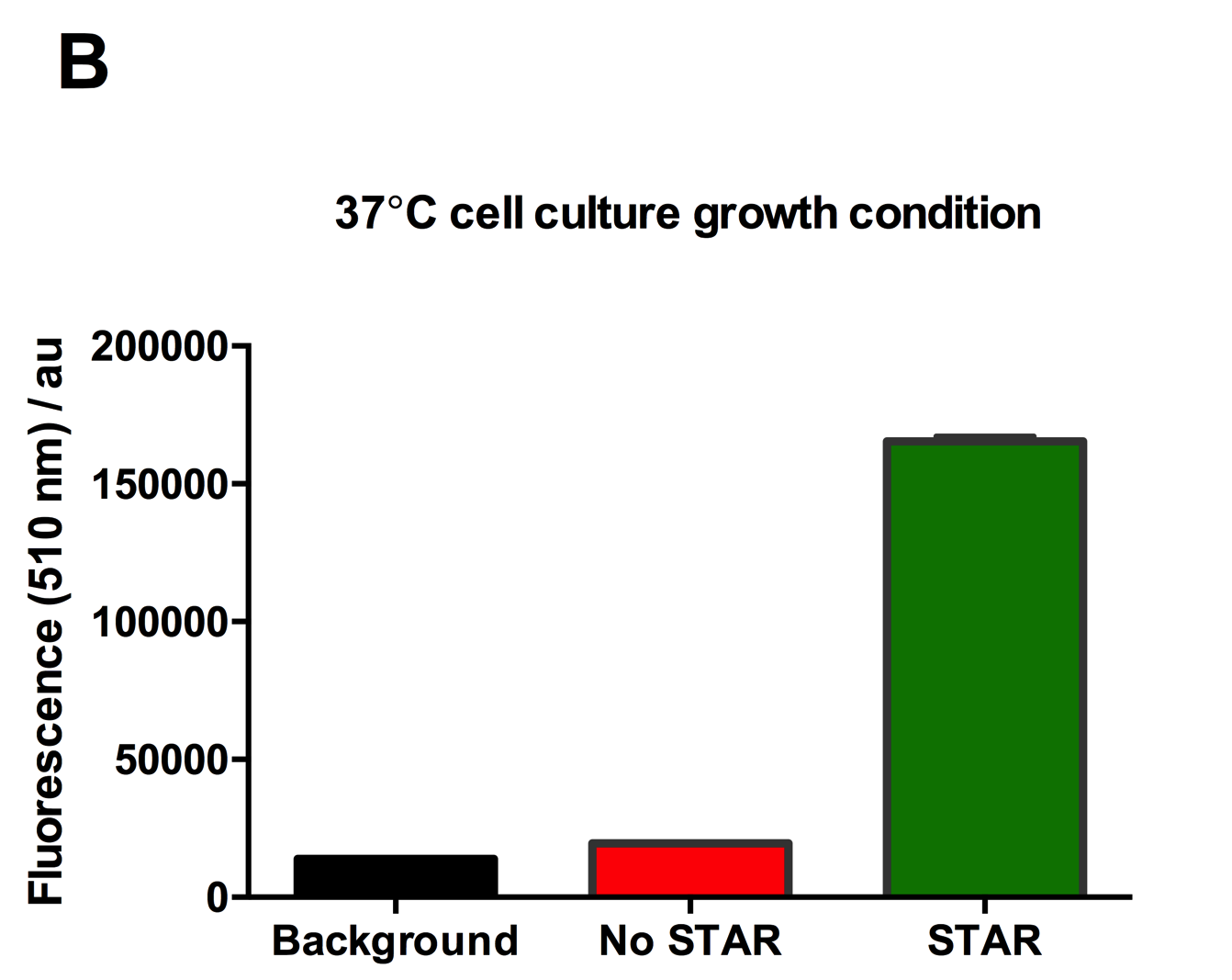
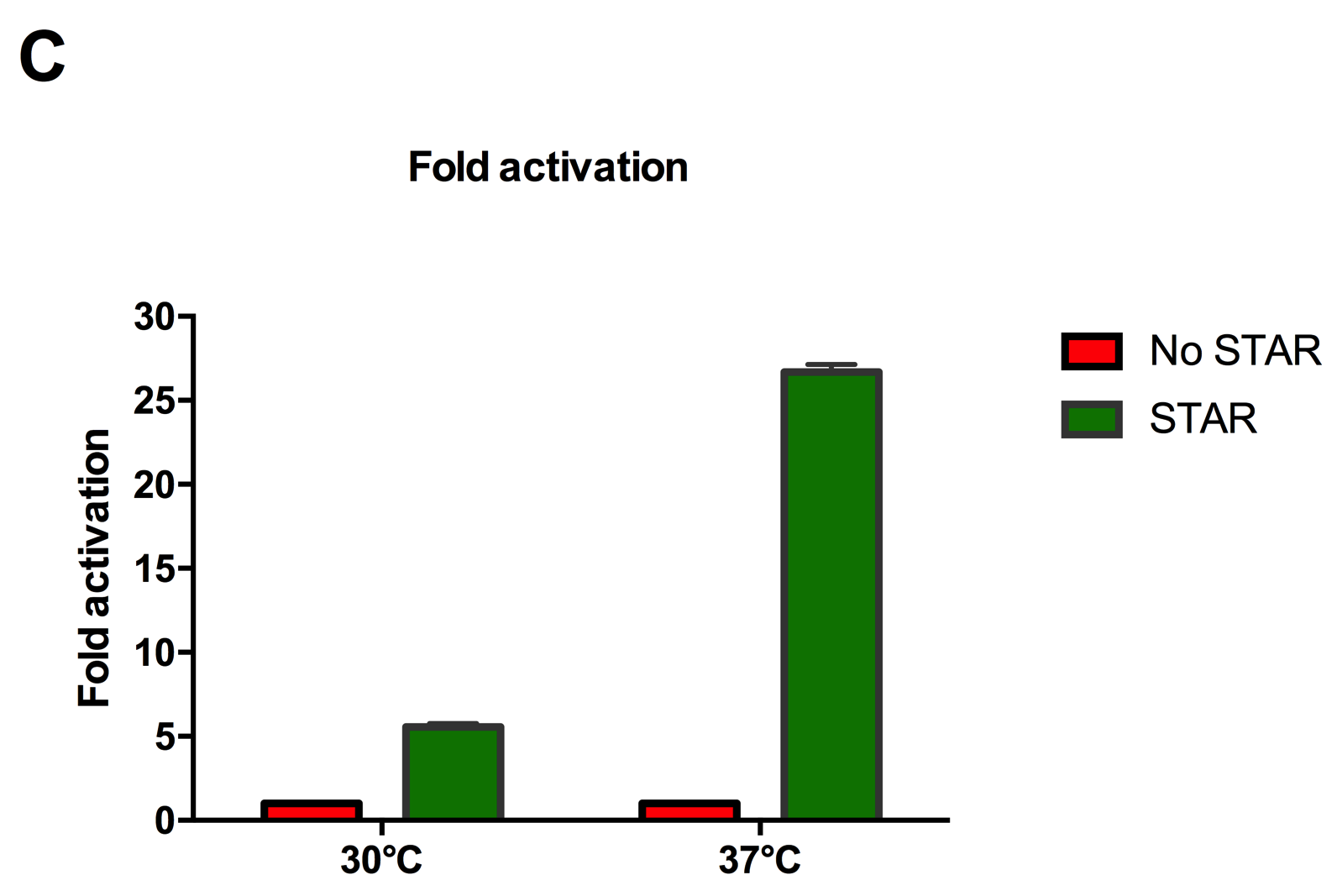
| + | [[File:IC16_STAR2Agraph.png|300px|]][[File:IC16_STAR2B.png|300px|]][[File:IC16_STAR2C.png|345px|]] | ||
| + | |||
| + | Figure 2: Characterisation of STAR system in TOP10 E. coli cells at different temperatures. (A) Cell culture fluorescence at 30°C (B) Cell culture fluorescence assay at 37°C. (C) Fold activation SFGFP expression in presence of STAR. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. The autofluorescence background control used is E. coli Top 10 cells with no reporter plasmid. Error bars represent standard deviation from 3 technical repeats. | ||
| + | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K1893013 SequenceAndFeatures</partinfo> | <partinfo>BBa_K1893013 SequenceAndFeatures</partinfo> | ||
Revision as of 15:58, 23 October 2016
Small transcription activating RNA (STAR)
This is the STAR construct with its own transcription terminator t500. When transcribed, it produces the STAR-antisense RNA (pAD1.A5), which can bind to the sense STAR-target attenuator sequence (pAD1.S5). Thus, only in the presence of STAR-antisense, transcription and expression of coding sequences downstream to the STAR-target is activated.
Usage and Biology
Figure 1: Characterisation of STAR system in TOP10 E. coli cells. (A) Normalised fluorescence monitored over time for cell lines incorporating the STAR system in the absence or presence of transcribed STAR molecules (B) Normalised endpoint fluorescence (100 minutes) for cell lines in the absence or presence of STAR molecules. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. Normalised fluorescence was calculated by dividing fluorescent signal by the O.D.600 value of the culture. Background was determined by the use of DH10B cells with no plasmid transformed. Error bars represent standard deviation from 3 technical repeats.
Figure 2: Characterisation of STAR system in TOP10 E. coli cells at different temperatures. (A) Cell culture fluorescence at 30°C (B) Cell culture fluorescence assay at 37°C. (C) Fold activation SFGFP expression in presence of STAR. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. The autofluorescence background control used is E. coli Top 10 cells with no reporter plasmid. Error bars represent standard deviation from 3 technical repeats.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]