Difference between revisions of "Part:BBa K777001"
(→Usage and Biology) |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K777001 short</partinfo> | <partinfo>BBa_K777001 short</partinfo> | ||
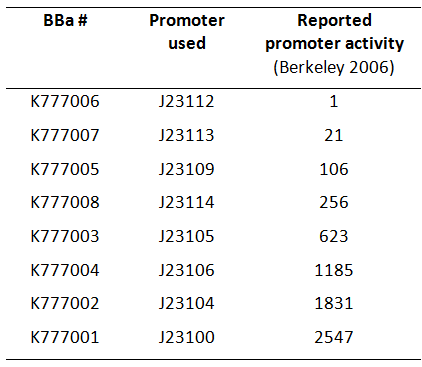
| − | [[Image:Table_K777001-K777008_new.png|thumb| | + | [[Image:Table_K777001-K777008_new.png|thumb|300px|right|'''Fig. 1:''' Overview of BioBricks containing the ''Tar'' gene downstream of Anderson promoters. (Activity according to Berkeley 2006)]] |
The transmembrane chemoreceptor Tar of <i>E. coli</i> mediates chemotaxis towards aspartate and exists as a functional homodimer. | The transmembrane chemoreceptor Tar of <i>E. coli</i> mediates chemotaxis towards aspartate and exists as a functional homodimer. | ||
| − | Here we used a set of 8 constitutive [https://parts.igem.org/Part:BBa_J23100 Anderson promoters] from the 2006 Berkeley group to test how different levels of constitutive ''Tar'' expression affect the chemotaxis of ''E. coli''. | + | Here, we used a set of 8 constitutive [https://parts.igem.org/Part:BBa_J23100 Anderson promoters] from the 2006 Berkeley group to test how different levels of constitutive ''Tar'' expression affect the chemotaxis of ''E. coli'' (Fig. 1). |
| − | <br> <br> <br> | + | <br><br> |
| − | <br> <br> <br> | + | The transcriptional activity of the constructs K777001-K777008 was quantified via quantitative real-time PCR. The results of this experiment can be found in the [https://parts.igem.org/Part:BBa_J23100:Experience#User_Reviews experience section] of the according Anderson promoters. Our data were compared to the activity of the promoters that can be found on the main page of [https://parts.igem.org/Part:BBa_J23100 Anderson promoters].<br> The respective raw dataset can be found in our Team-wiki [http://2012.igem.org/Team:Goettingen/Project/Computational_Data#Raw_Datasets here]. |
| − | <br> <br> | + | <br> <br><br> <br> <br><br> <br> <br> |
| − | <br> <br> <br> | + | |
| Line 16: | Line 15: | ||
* Here is the complete sequence of this part as an [https://static.igem.org/mediawiki/2012/f/fc/Tar_receptor_under_the_control_of_constitutive_promoter.zip ApE file]. | * Here is the complete sequence of this part as an [https://static.igem.org/mediawiki/2012/f/fc/Tar_receptor_under_the_control_of_constitutive_promoter.zip ApE file]. | ||
<br> | <br> | ||
| − | [[Image:Tar Bindingsite01labled.png|thumb| | + | ====Chemotaxis functionality==== |
| − | Here, we mutated this part at in total 5 amino acid sites which are important for ligand binding. The mutated sites are the nucleotides for the amino acids at position 69, 73, 149, 150 and 154 | + | [[Image:Gruppe3_Figure02neu.png|500px|thumb|right|'''Fig. 2:''' Promoter comparison through a chemotaxis assay.]] |
| − | + | The different promoter constructs K777001-K777008 were all tested for chemotaxis towards aspartate (Fig. 2). | |
| − | + | Eight promoters of the „Anderson“ collection were cloned in front of the gene coding for the Tar receptor inside the pSB1C3 backbone. Here, we compared the swimming behavior of these strains in chemotaxis assay with M9-swimming agar plates. The chemotaxis assay was conducted as described in the methods section. L-aspartate served as the attractant in the central Whatman paper. The numbers next to each halo correspond to the promoter activities reported by the Berkley University in 2006. <b>(A)</b> The constructs were transformed in the Δtar strain. The picture was taken after 3 days incubation at 33 °C. The halos of the strongest promoters (J23100, x2547 and J23104, x1831) had a slightly bigger radius than the other promoter constructs. In addition, slight chemotaxis could be observed for these strains. The drop of the construct J23106 was smeared during the application and is therefore not evaluable. <b>(B)</b> The constructs were transformed in the BL21 strain, that naturally expresses the Tar receptor. The picture was taken after 1 day incubation at 33 °C. In general, halo size did not vary strongly. The halos of the strongest promoters (J23100, x2547 and J23104, x1831) had the biggest radius. The halo of J23106 (x1185) showed the smallest radius, although this promoter has the third stronges activity. Chemotaxis could be observed for every strain. | |
| − | + | <br><br><br> | |
| − | + | ====Mutation library==== | |
| − | + | [[Image:Tar Bindingsite01labled.png|thumb|500px|left|'''Fig. 3:''' PYMOL images of Tar with aspartate in its binding pocket (PDB ID: 1WAT). The mutated amino acid residues involved in ligand binding are labeled.]] | |
| + | Here, we mutated this part at in total 5 amino acid sites which are important for ligand binding (Fig. 3). The mutated sites are the nucleotides for the amino acids at position 69, 73, 149, 150 and 154. | ||
<br> | <br> | ||
<br> | <br> | ||
| Line 29: | Line 29: | ||
<br> | <br> | ||
<br> | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | [[Image:Sequencing2.png|thumb|500px|left|'''Fig. 4:''' Sequencing chromatogram excerpt of the generated mutated chemoreceptor Tar_QC library in comparison to the original sequence Tar_QC. On the left, the sequencing chromatogram excerpt represents the original sequence, the right side shows the mutated sequence chromatogram. Sequencing results (top) with mutations for the amino acids at position 149, 150 and 154 and (bottom) with mutations at 69 and 73. Inserted mutations are indicated by the triplets consisting of N and K, whereby K stands for guanine or thymine, N for any possible base. Only in the mutated regions no distinct peaks are present. G: guanine, C: cytosine, A: adenine, T: thymine.]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | The sequencing chromatogram (Fig. 4) of the generated chemoreceptor Tar_QC library revealed a functional mutagenesis. Randomly picked clones indicated a diversity of 2x 10<sup>5</sup> clones. | ||
| + | <br> | ||
| + | The constructed library was tested in a library selection experiment in which seven new chemical attractants were tested for chemotaxis of the novel generated receptors. | ||
| + | As similar chemoreceptors were found to recognize the same respective chemicals, this is evidence for a functional part. | ||
| + | <br> | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | For more information on the generation of the mutation Tar_QC library and the library selection experiment visit our [http://2012.igem.org/Team:Goettingen/Notebook/Results#.233_-_Chemoreceptor_Library results page]. | ||
| + | <br> | ||
| + | <br> | ||
| + | ===Contributions=== | ||
| + | <I>Technion team 2016</I> | ||
| + | <br><br> | ||
| + | Adding a RBS and a terminator making a well functional device ( K1992004) allowing the expression of Tar chemoreceptor and chemotaxis response (link), | ||
| + | The improved BioBrick ( K1992004) contain the promoter (J23100), a strong RBS (B0034), the Tar coding sequence (K777000) and a double terminator (B0015). in order to test the device it was cloned to chemoreceptor free strain (UU1250) and tested for chemotaxis ability using swarming assay. | ||
| + | As can be seen in figure 1 the cells expressing the Tar chemoreceptor create a halo indicating a functional chemotaxis result, which compared to the negative and positive controls. The halo can be seen in a matter of hours ensuring that the halo is a result of a chemotaxis response. | ||
| + | |||
| + | [[File:T--Technion Israel--tar1swarming.jpg|600px|thumb|center|Fig1. (a) Tar expression in UU1250 strain, resulting a halo indicating a functional chemotaxis response. (b) Negative control- UU1250 strain w/o the Tar expression plasmid. (c) positive control - ΔZ strain expressing all chemoreceptors. | ||
| + | ]] | ||
| + | |||
| + | The part was successfully sequenced (fig2) ensuring the present of the promoter, RBS, Tar receptor and terminator. | ||
| + | |||
| + | [[File:RBS alignment.png|600px|thumb|center|Fig 2. The alignment of the sequencing results in compared to the designed sequence - alignment was done using snapgene software. | ||
| + | ]] | ||
| + | |||
| + | |||
<!-- --> | <!-- --> | ||
| − | <span class='h3bb'>Sequence and Features</span> | + | <span class='h3bb'>'''Sequence and Features'''</span> |
<partinfo>BBa_K777001 SequenceAndFeatures</partinfo> | <partinfo>BBa_K777001 SequenceAndFeatures</partinfo> | ||
<br> | <br> | ||
| − | [[Image:K777001_in_pSB1C3.png|thumb|240px|frame|left|'''Fig. | + | [[Image:K777001_in_pSB1C3.png|thumb|240px|frame|left|'''Fig. 4:''' Plasmid map of K777001 in pSB1C3]] |
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 06:57, 22 October 2016
Tar receptor under the control of constitutive promoter J23100
The transmembrane chemoreceptor Tar of E. coli mediates chemotaxis towards aspartate and exists as a functional homodimer.
Here, we used a set of 8 constitutive Anderson promoters from the 2006 Berkeley group to test how different levels of constitutive Tar expression affect the chemotaxis of E. coli (Fig. 1).
The transcriptional activity of the constructs K777001-K777008 was quantified via quantitative real-time PCR. The results of this experiment can be found in the experience section of the according Anderson promoters. Our data were compared to the activity of the promoters that can be found on the main page of Anderson promoters.
The respective raw dataset can be found in our Team-wiki [http://2012.igem.org/Team:Goettingen/Project/Computational_Data#Raw_Datasets here].
Usage and Biology
This part can be used for chemotaxis assays.
- Here is the complete sequence of this part as an ApE file.
Chemotaxis functionality
The different promoter constructs K777001-K777008 were all tested for chemotaxis towards aspartate (Fig. 2).
Eight promoters of the „Anderson“ collection were cloned in front of the gene coding for the Tar receptor inside the pSB1C3 backbone. Here, we compared the swimming behavior of these strains in chemotaxis assay with M9-swimming agar plates. The chemotaxis assay was conducted as described in the methods section. L-aspartate served as the attractant in the central Whatman paper. The numbers next to each halo correspond to the promoter activities reported by the Berkley University in 2006. (A) The constructs were transformed in the Δtar strain. The picture was taken after 3 days incubation at 33 °C. The halos of the strongest promoters (J23100, x2547 and J23104, x1831) had a slightly bigger radius than the other promoter constructs. In addition, slight chemotaxis could be observed for these strains. The drop of the construct J23106 was smeared during the application and is therefore not evaluable. (B) The constructs were transformed in the BL21 strain, that naturally expresses the Tar receptor. The picture was taken after 1 day incubation at 33 °C. In general, halo size did not vary strongly. The halos of the strongest promoters (J23100, x2547 and J23104, x1831) had the biggest radius. The halo of J23106 (x1185) showed the smallest radius, although this promoter has the third stronges activity. Chemotaxis could be observed for every strain.
Mutation library
Here, we mutated this part at in total 5 amino acid sites which are important for ligand binding (Fig. 3). The mutated sites are the nucleotides for the amino acids at position 69, 73, 149, 150 and 154.

The sequencing chromatogram (Fig. 4) of the generated chemoreceptor Tar_QC library revealed a functional mutagenesis. Randomly picked clones indicated a diversity of 2x 105 clones.
The constructed library was tested in a library selection experiment in which seven new chemical attractants were tested for chemotaxis of the novel generated receptors.
As similar chemoreceptors were found to recognize the same respective chemicals, this is evidence for a functional part.
For more information on the generation of the mutation Tar_QC library and the library selection experiment visit our [http://2012.igem.org/Team:Goettingen/Notebook/Results#.233_-_Chemoreceptor_Library results page].
Contributions
Technion team 2016
Adding a RBS and a terminator making a well functional device ( K1992004) allowing the expression of Tar chemoreceptor and chemotaxis response (link),
The improved BioBrick ( K1992004) contain the promoter (J23100), a strong RBS (B0034), the Tar coding sequence (K777000) and a double terminator (B0015). in order to test the device it was cloned to chemoreceptor free strain (UU1250) and tested for chemotaxis ability using swarming assay.
As can be seen in figure 1 the cells expressing the Tar chemoreceptor create a halo indicating a functional chemotaxis result, which compared to the negative and positive controls. The halo can be seen in a matter of hours ensuring that the halo is a result of a chemotaxis response.
The part was successfully sequenced (fig2) ensuring the present of the promoter, RBS, Tar receptor and terminator.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 1323
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 152