Difference between revisions of "Part:BBa K2086001"
(→Biology and Our Application) |
(→SerA Supplementation) |
||
| Line 30: | Line 30: | ||
===SerA Supplementation=== | ===SerA Supplementation=== | ||
| − | [[Image:SerASupplementationGrowthCurve.jpg|450px|thumb|left|'''Figure 2:''' The growth curve of <i>E. coli</i> JW2880 complemented with a plasmid (SerA + native promoter). OD600 measured every 12.5 minutes to indicate cell growth.]] | + | [[Image:SerASupplementationGrowthCurve.jpg|450px|thumb|left|'''Figure 2:''' The growth curve of <i>E. coli</i> JW2880 complemented with a plasmid (SerA + native promoter). OD600 measured every 12.5 minutes to indicate cell growth grown in our M9 minimal medium.]] |
| − | [[Image: | + | [[Image:QualitativeSerASupplemenation.jpg|450px|thumb|Right|'''Figure 3:''' Qualitative results from testing our composite biobrick <partinfo>K2086002</partinfo>. Both tubes contain <i>JW2880</i>, complemented with our PyeaR-SerA kill switch, grown anaerobically in our M9 minimal medium. The left bottle contains no potassium nitrate, while the right contains 4 mM potassium nitrate.]] |
| − | Before | + | Before interpreting the results from our composite part including SerA, it's important to first consider whether our selected auxotroph (JW2880) was able to be rescued with a supplemental SerA gene. By complementing our auxotroph with a plasmid containing SerA and its native promoter, the growth curve in <b>Figure 2</b> was obtained. |
| − | + | The ability of our isolated SerA gene to rescue our auxotroph was confirmed by testing our composite biobrick kill switch (<part info>K2086002</partinfo>), which expresses the SerA gene when nitrate concentrations reach a certain threshold. The qualitative results from one of these experiments are pictured in <b>Figure 4</b>. | |
===Plasmid Synthesis=== | ===Plasmid Synthesis=== | ||
Revision as of 22:29, 16 October 2016
Serine Repeat Antigen (SerA)
The E. coli serA gene encodes the D-3-phosphoglycerate dehydrogenase, which catalyzes the first committed step in the biosynthesis of serine. Serine is an essential amino acid for E. coli growth in minimal medium. Deletion of the serA gene leads to a serine auxotroph, which can be rescued either by the expression of a protein with the same catalytic activity as SerA or by the addition of serine in the growth media.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 293
- 23INCOMPATIBLE WITH RFC[23]Unknown
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Biology and Our Application
Serine is an amino acid produced in E. coli K12 through the metabolic pathway shown in Figure 1. The SerA gene codes for D-3-Phosphoglycerate Dehydrogenase, the enzyme responsible for catalyzing the committed step of serine biosynthesis. Without SerA, E. coli are unable to grow without sufficient supplementation of other amino acids [1].
Taking advantage of the bacteria's dependence on serine, we planned to create a safety kill switch by controlling the production of the amino acid. By obtaining an auxotrophic strain missing the SerA strain (JW2880 [2]) we were able to create a complement SerA plasmid to rescue the strain when grown in media without supplementary amino acids. Our kill-switch (BBa_K2086002) was incorporated into our final project design.
[1]. PAULA D. RAVNIKAR AND RONALD L. SOMERVILLE: Genetic Characterization of a Highly Efficient Alternate Pathway of Serine Biosynthesis in Escherichia coli. http://jb.asm.org/content/169/6/2611.full.pdf
[2] Coli Generic Stock Center: JW2880. http://cgsc.biology.yale.edu/Strain.php?ID=108515
Isolation from Genomic DNA
SerA Supplementation

Before interpreting the results from our composite part including SerA, it's important to first consider whether our selected auxotroph (JW2880) was able to be rescued with a supplemental SerA gene. By complementing our auxotroph with a plasmid containing SerA and its native promoter, the growth curve in Figure 2 was obtained. The ability of our isolated SerA gene to rescue our auxotroph was confirmed by testing our composite biobrick kill switch (<part info>K2086002</partinfo>), which expresses the SerA gene when nitrate concentrations reach a certain threshold. The qualitative results from one of these experiments are pictured in Figure 4.
Plasmid Synthesis
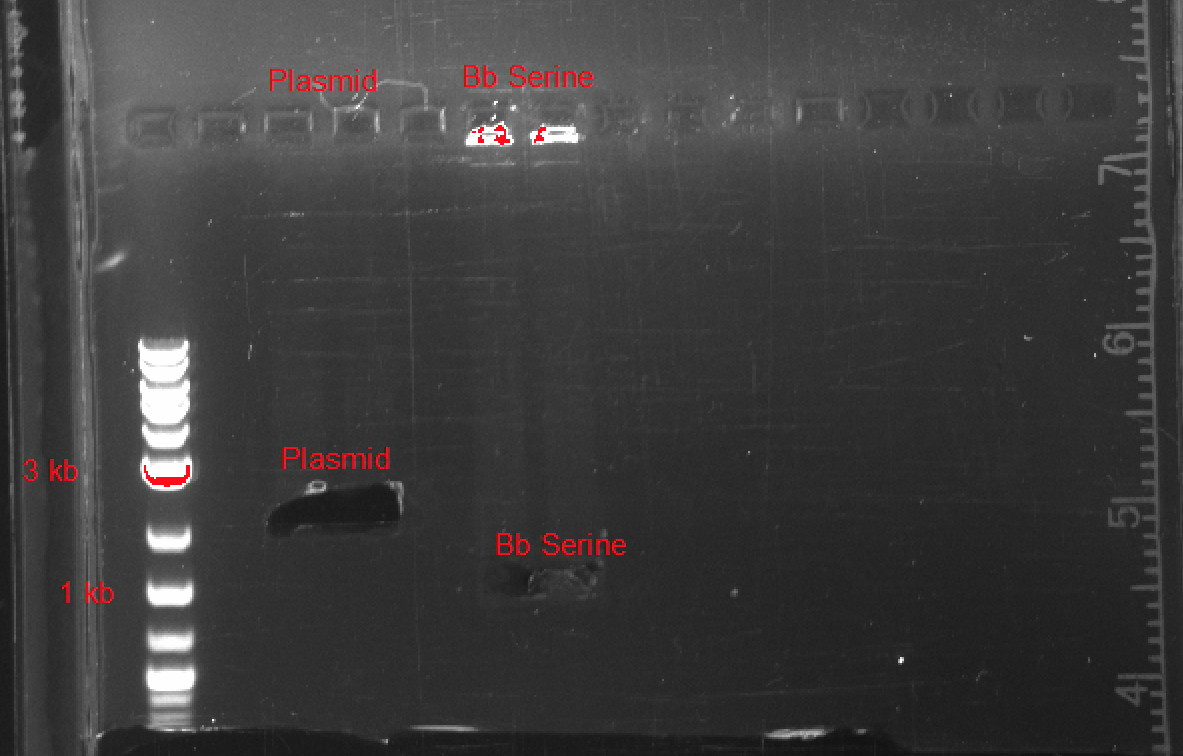
After testing, we were able to synthesize a basic SerA ORF plasmid, biobrick compatible with a wide variety of plasmids, promoters, and ribosome binding sites. In Figure 3, you can see the results of our gel electrophoresis, used to ligate the two components of our plasmid:
- pSB1C3 : 2070 bp
- SerA : 1233 bp