Difference between revisions of "Part:BBa K1499004"
(→Usage and Biology) |
(→Results) |
||
| Line 25: | Line 25: | ||
===Results=== | ===Results=== | ||
We plan to assay the functionality of this part using a GFP construct inside a cell with the AviTag/OmpA surface complex. We will purify and apply our CBD/Streptavidin fusion protein to a cellulosic surface, followed by the above transformed cells, and attempt to wash off the cells using an isotonic solution. If the level of fluorescence does not change, we can assume our system has been successful. | We plan to assay the functionality of this part using a GFP construct inside a cell with the AviTag/OmpA surface complex. We will purify and apply our CBD/Streptavidin fusion protein to a cellulosic surface, followed by the above transformed cells, and attempt to wash off the cells using an isotonic solution. If the level of fluorescence does not change, we can assume our system has been successful. | ||
| + | |||
| + | The 2016 Stanford-Brown iGEM Team purified this linker protein and used it to create a BioDevice. Used in tandem with a biotinylated fluorophore, this CBD/Streptavidin fusion protein served as a linker between cellulose paper and the fluorophore-quencher biosensor described <a href="http://2016.igem.org/Team:Stanford-Brown/SB16_BioSensor_FQsensor">here.</a> | ||
| + | |||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Revision as of 07:27, 13 October 2016
Cellulose binding domains with streptavidin domain generator
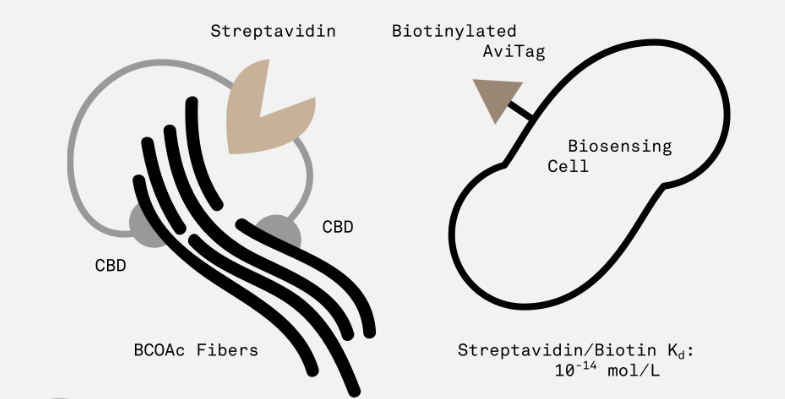
This part encodes a protein with two cellulose-binding domains on either end joined by a streptavidin domain.
Usage and Biology
The idea behind this part is that each domain binds to a single cellulose fiber, thus providing a way to cross-link and strengthen cellulose polymers. In addition, the streptavidin domain allows for the modular addition of sensor cells with a biotinylated AviTag peptide (expressed on the surface of the cell by appending it to outer membrane protein OmpA).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 703
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 304
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 651
Characterization
Verification of Part
The part was sequence verified before submission to the registry with two reads using VF2 and VR.
Results
We plan to assay the functionality of this part using a GFP construct inside a cell with the AviTag/OmpA surface complex. We will purify and apply our CBD/Streptavidin fusion protein to a cellulosic surface, followed by the above transformed cells, and attempt to wash off the cells using an isotonic solution. If the level of fluorescence does not change, we can assume our system has been successful.
The 2016 Stanford-Brown iGEM Team purified this linker protein and used it to create a BioDevice. Used in tandem with a biotinylated fluorophore, this CBD/Streptavidin fusion protein served as a linker between cellulose paper and the fluorophore-quencher biosensor described <a href="http://2016.igem.org/Team:Stanford-Brown/SB16_BioSensor_FQsensor">here.</a>