Difference between revisions of "Part:BBa K1689004"
| Line 2: | Line 2: | ||
<partinfo>BBa_K1689004 short</partinfo> | <partinfo>BBa_K1689004 short</partinfo> | ||
| − | Nluc398 fusion protein ORF | + | Nluc398-FRB fusion protein ORF |
Firefly (<I>Photinus pyralis</I>) luciferase can be split to N-terminal (Nluc) and C-terminal (Cluc) fragments and each of them is inactive. When they two reassembled non-covalently, the enzymatic activity would be reconstituted and the recovered luciferase is able to oxidize luciferin and produce detectable bioluminescence. Currently there are different combinations of split fragments, among which Nluc 416 / Cluc 398 and Nluc 398/ Cluc 394 are widely used[1]. | Firefly (<I>Photinus pyralis</I>) luciferase can be split to N-terminal (Nluc) and C-terminal (Cluc) fragments and each of them is inactive. When they two reassembled non-covalently, the enzymatic activity would be reconstituted and the recovered luciferase is able to oxidize luciferin and produce detectable bioluminescence. Currently there are different combinations of split fragments, among which Nluc 416 / Cluc 398 and Nluc 398/ Cluc 394 are widely used[1]. | ||
Revision as of 14:53, 27 September 2015
Coding sequence of Nluc398-FRB
Nluc398-FRB fusion protein ORF
Firefly (Photinus pyralis) luciferase can be split to N-terminal (Nluc) and C-terminal (Cluc) fragments and each of them is inactive. When they two reassembled non-covalently, the enzymatic activity would be reconstituted and the recovered luciferase is able to oxidize luciferin and produce detectable bioluminescence. Currently there are different combinations of split fragments, among which Nluc 416 / Cluc 398 and Nluc 398/ Cluc 394 are widely used[1].
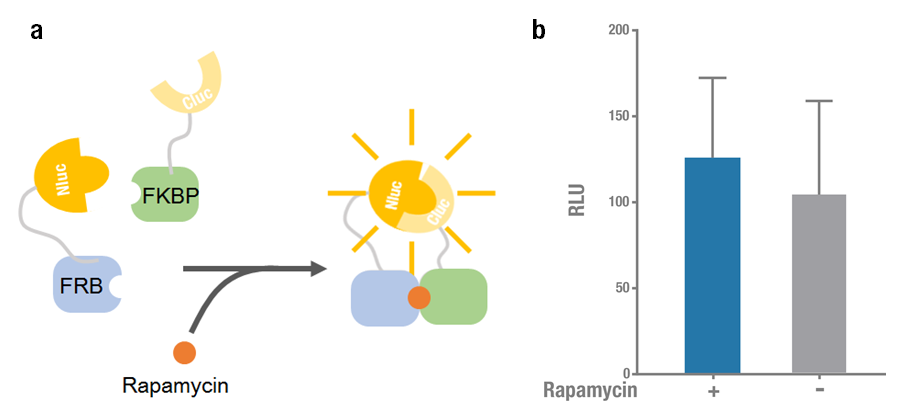
Rapamycin-binding domain (FRB) of human mTOR (mammalian Target of Rapamycin) binds with high affinity to FK-506-binding protein 12 (FKBP). Previously Raik Gruenberg had already designed the part BBa_J18926, containing the coding sequence of FRB. Rapamycin is able to induce the dimerization to form a FRB-rapamycin-FKBP complex[2]. This protein-protein interaction can be visualized by split luciferase[3]. FRB and FKBP are fused to Nluc and Cluc respectively, and adding rapamycin can induce the approaching and reconstitution of split luciferase (Figure 1a).
2015 Peking iGEM improved the previous part BBa_J18926, they fused Nluc 398 to N terminus of FRB (Nluc 398-FRB, BBa_K1689004) and combined it with FKBP-Cluc 394 (BBa_K1689006) to validate the functional reconstitution of split luciferase. However, compared with Nluc 416/ Cluc 398, the bioluminescence intensity didn't increase significantly after rapamycin was added (Figure 1). Therefore we discarded them and chose Nluc 416/Cluc 398 as our split luciferase in the project (See BBa_K1689003 or BBa_K1689005).
Figure 1. Rapamycin-induced N-luc-FRB/FKBP-C-luc complementation. (a) The working mechanism of rapamycin induced dimerization. The interacting protein partners (FRB & FKBP) get closer and dimerize soon after rapamycin is added (40nM) [3], thus to reconstitute the enzymatic activity of luciferase. (b) The experimental data. Error bars denote s.d.; n=3.
References
1. Ramasamy Paulmurugan, Sanjiv S. Gambhir. Firefly Luciferase Enzyme Fragment Complementation for Imaging in Cells and Living Animals. Anal Chem. 2005 March 1; 77(5): 1295–1302.
2. Rivera, V. M., T. Clackson, S. Natesan et al. A humanized system for pharmacologic control of gene expression. Nat. Med. 1996. 2:1028–1032.
3. Ramasamy Paulmurugan, Sanjiv S. Gambhir. Combinatorial