Difference between revisions of "Part:BBa K1694007"
| Line 4: | Line 4: | ||
<h1>'''Introduction'''</h1> | <h1>'''Introduction'''</h1> | ||
<p style="font-size:120%">'''FadL'''</p> | <p style="font-size:120%">'''FadL'''</p> | ||
| − | |||
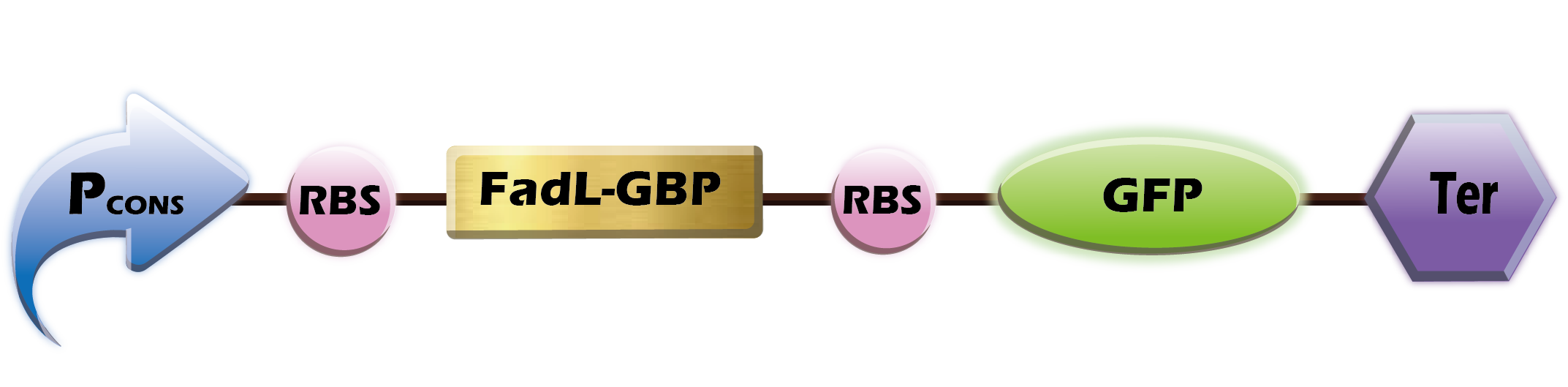
[[File:FGB.png|200px|thumb|right|'''Fig.1''' FadL-GBP]] | [[File:FGB.png|200px|thumb|right|'''Fig.1''' FadL-GBP]] | ||
The FadL protein (48.8 kDa) is an outer membrane protein of the ''E. coli'', which plays an important role in the uptake of exogenous long-chain fatty acid. The FadL protein has twenty antiparallel β-strands, which form a β-barrel structure and are connected by 9 internal and 10 external loops. Due to the β-structure, the FadL protein is able to span the outer membrane multiple times to form a long-chain fatty acid-specific channel. With these properties, the truncated FadL protein can be used as a novel anchoring motif for display of proteins on the ''E. coli'' cell surface<sup>[1]</sup>. | The FadL protein (48.8 kDa) is an outer membrane protein of the ''E. coli'', which plays an important role in the uptake of exogenous long-chain fatty acid. The FadL protein has twenty antiparallel β-strands, which form a β-barrel structure and are connected by 9 internal and 10 external loops. Due to the β-structure, the FadL protein is able to span the outer membrane multiple times to form a long-chain fatty acid-specific channel. With these properties, the truncated FadL protein can be used as a novel anchoring motif for display of proteins on the ''E. coli'' cell surface<sup>[1]</sup>. | ||
<br><br> | <br><br> | ||
| − | <p style="font-size:120%">'''GBP'''</p | + | <p style="font-size:120%">'''GBP'''</p> |
The gold binding polypeptide, abbreviated as GBP, is composed of three amino acid sequence repetition of MHGKTQATSGTIQS. This ''E. coli'' cell-surface display system has developed for several years<sup>[2]</sup>. The recent computation study demonstrated that multiple repeats of GBP have higher affinity to gold surface, because a high degree of conformational flexibility can make the binding much stronger. | The gold binding polypeptide, abbreviated as GBP, is composed of three amino acid sequence repetition of MHGKTQATSGTIQS. This ''E. coli'' cell-surface display system has developed for several years<sup>[2]</sup>. The recent computation study demonstrated that multiple repeats of GBP have higher affinity to gold surface, because a high degree of conformational flexibility can make the binding much stronger. | ||
The connection mechanism between GBP and gold metal plane is still unknown. By using the concept of '''molecular dynamics''' (MD), GBP, with the structure of antiparallel β-sheet, is shown to attach to gold surface via OH-binding. It seems that the hydroxyl, as well as the amine ligands on GBP, can recognize the atomic lattice structure of gold for the alignment of the molecule along the variants of a six-fold axis on the Au (111) surface<sup>[3]</sup>. In addition, another study also states that water molecules played an important role in the surface recognition by the side chain and hydration layer of the peptides to reach the better binding <sup>[4]</sup>. | The connection mechanism between GBP and gold metal plane is still unknown. By using the concept of '''molecular dynamics''' (MD), GBP, with the structure of antiparallel β-sheet, is shown to attach to gold surface via OH-binding. It seems that the hydroxyl, as well as the amine ligands on GBP, can recognize the atomic lattice structure of gold for the alignment of the molecule along the variants of a six-fold axis on the Au (111) surface<sup>[3]</sup>. In addition, another study also states that water molecules played an important role in the surface recognition by the side chain and hydration layer of the peptides to reach the better binding <sup>[4]</sup>. | ||
Revision as of 07:49, 22 September 2015
FadL-GBP
Introduction
FadL
The FadL protein (48.8 kDa) is an outer membrane protein of the E. coli, which plays an important role in the uptake of exogenous long-chain fatty acid. The FadL protein has twenty antiparallel β-strands, which form a β-barrel structure and are connected by 9 internal and 10 external loops. Due to the β-structure, the FadL protein is able to span the outer membrane multiple times to form a long-chain fatty acid-specific channel. With these properties, the truncated FadL protein can be used as a novel anchoring motif for display of proteins on the E. coli cell surface[1].
GBP
The gold binding polypeptide, abbreviated as GBP, is composed of three amino acid sequence repetition of MHGKTQATSGTIQS. This E. coli cell-surface display system has developed for several years[2]. The recent computation study demonstrated that multiple repeats of GBP have higher affinity to gold surface, because a high degree of conformational flexibility can make the binding much stronger. The connection mechanism between GBP and gold metal plane is still unknown. By using the concept of molecular dynamics (MD), GBP, with the structure of antiparallel β-sheet, is shown to attach to gold surface via OH-binding. It seems that the hydroxyl, as well as the amine ligands on GBP, can recognize the atomic lattice structure of gold for the alignment of the molecule along the variants of a six-fold axis on the Au (111) surface[3]. In addition, another study also states that water molecules played an important role in the surface recognition by the side chain and hydration layer of the peptides to reach the better binding [4].
Reference:
[1] Display of Bacterial Lipase on the Escherichia coli Cell Surface by Using FadL as an Anchoring Motif and Use of the Enzyme in Enantioselective Biocatalysis, Seung Hwan Lee1, Jong-Il Choi1, Si Jae Park1,†, Sang Yup Lee1,2,* and Byoung Chul Park3(2004)
[2] Molecular characterization of a prokaryotic polypeptide sequence that catalyzes Au crystal formation, John L. Kulp III,a Mehmet Sarikayab and John Spencer Evans, Journal of Materials Chemistry(2004)
[3] Assembly of Gold-Binding Proteins for Biomolecular Recognition, Zareie HM1,2* and Sarikaya M3, Austin Journal of Biosensors & Bioelectronics (2015)
[4] Thermodynamics of Engineered Gold Binding Peptides: Establishing the Structure−Activity Relationships, Urartu Ozgur Safak Seker, Brandon Wilson, John L. Kulp,§ John S. Evans, Candan Tamerler, and Mehmet Sarikaya(2014)
Experiment
After receiving the DNA sequences from the gene synthesis company, we placed the FadL-GBP genes into pSB1C3 backbones and conducted PCR experiments to ascertain the size of each FadL-GBP sequence. The length of FadL-GBP DNA sequence is around 1200~1350 bp. Therefore, in the PCR experiment, the expected size of FadL-GBP products should be 1450~1600 bp. Fig.2 affirms the size of the FadL-GBP and proves that we have successfully ligated the FadL-GBP sequence onto the ideal backbone.
Application
For more direct observation of the result, our E. coli is created to produce green fluorescence at the same time. To make the comparison of the binding efficiency, we selected the genetic sequence, Pcons + RBS + GFP + Ter, as the negative control group. Since there are no known existence of a fully-expressed GBP phenomenon, there are no positive control in our experiment.
According to the pictures shown above, we can see that there are more E. coli with gold-binding polypeptide expression being observed on the gold chip. This indicates that the E. coli with gold-binding polypeptide can attach onto the gold chip more effectively and efficiently than our negative control group.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 438
Illegal BamHI site found at 924 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 214
Illegal NgoMIV site found at 706
Illegal NgoMIV site found at 1131
Illegal AgeI site found at 847 - 1000COMPATIBLE WITH RFC[1000]