Difference between revisions of "Part:BBa K1383000"
(→Characterization) |
|||
| (24 intermediate revisions by 3 users not shown) | |||
| Line 7: | Line 7: | ||
Characterization of the RBS sequences was accomplished using the red fluorescent protein, mCherry as a reporter. Qualitative analysis was evaluated by visualization of the RFP and quantitative analysis was completed through use of a plate reader for reading fluorescence. | Characterization of the RBS sequences was accomplished using the red fluorescent protein, mCherry as a reporter. Qualitative analysis was evaluated by visualization of the RFP and quantitative analysis was completed through use of a plate reader for reading fluorescence. | ||
| − | |||
| − | |||
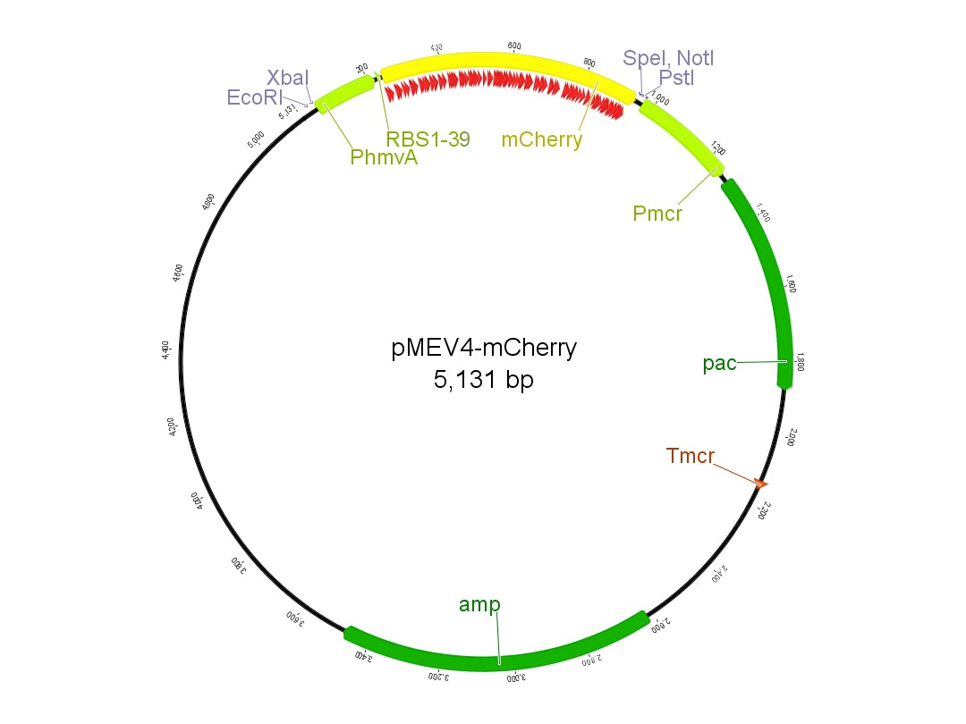
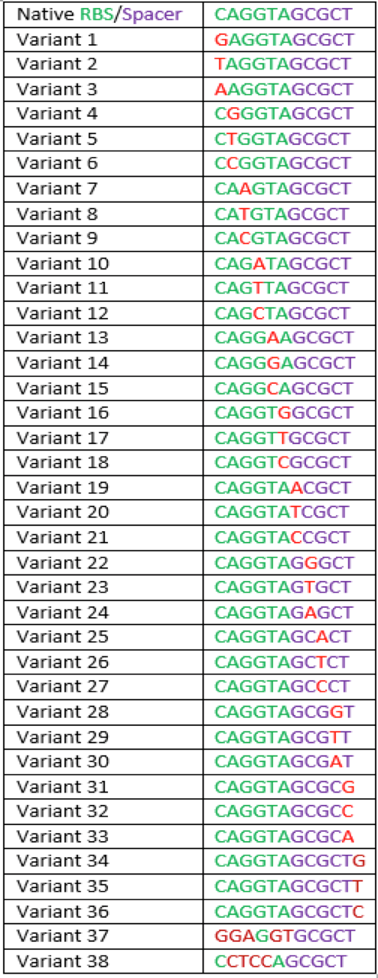
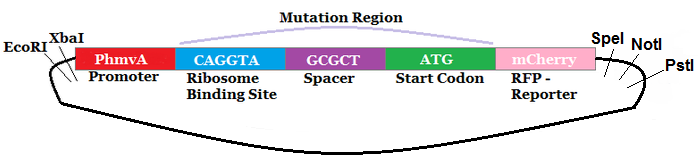
| − | The region labeled 'RBS 1-39' in the pMEV4-mCherry vector (figure 1) is the site immediately upstream of the mCherry gene that will be subject to variation. Specifically, we will be mutating the 6 base pairs of the RBS, the 5 of the spacer, and the first base of the start codon. We begin with the 'native' RBS, which is a known functional RBS sequence in methanogens and is typically used for creating synthetic parts. The RBS variants are created by making mutations on only one base at a time for every nucleotide different from the native (figure 2). We designed two additional RBS sequences based off the 16S rRNA data for <i>M. maripaludis</i>, termed theoretical 'perfect' and 'negative' (figure 2, #37 & #38, respectively). These RBS sequences were designed to have the theoretical greatest and worst possible affinities for the 16S ribosome. Figure 3 illustrates the specific sequence for the part described on this page, the 'native' RBS. | + | '''2013: Improving the Construct and Characterization of Fluorescent Reporters for use in Methanogens''' |
| + | |||
| + | In 2013, the UGA-Georgia team submitted the part [https://parts.igem.org/Part:BBa_K1138002 BBa_K1138002], named pAW50-mCherry. This construct, designed for use as a fluorescent reporter in methanogens, had a few issues. First, the part was not 100% biobrick compatible due to an internal restriction site in the mCherry gene. The characterization of the part was also inconsistent among samples, and overall unreproducible. To improve upon the previous part, we designed pMEV4-mCherry (BBa_K1383000, figure 1). The primary differences between this construct and the pAW50-mCherry of 2013 is that pMEV4-mCherry does not contain the internal restriction site and the selective resistance gene against puromycin, our antibiotic for use in <i>M. maripaludis</i>, has an independent promoter. Therefore, pMEV4-mCherry is 100% biobrick compatible, and can more reliably function under increased selective pressure. Improvements on characterization are elaborated more below. | ||
| + | |||
| + | The region labeled 'RBS 1-39' in the pMEV4-mCherry vector (figure 1) is the site immediately upstream of the mCherry gene that will be subject to variation. Specifically, we will be mutating the 6 base pairs of the RBS, the 5 of the spacer, and the first base of the start codon. We begin with the 'native' RBS ([https://parts.igem.org/Part:BBa_K1383000 BBa_K1383000], shown on this page), which is a known functional RBS sequence in methanogens and is typically used for creating synthetic parts. The RBS variants are created by making mutations on only one base at a time for every nucleotide different from the native (figure 2). We designed two additional RBS sequences based off the 16S rRNA data for <i>M. maripaludis</i>, termed theoretical 'perfect' and 'negative' (figure 2, #37 & #38, [https://parts.igem.org/Part:BBa_K1383001 BBa_K1383001] & [https://parts.igem.org/Part:BBa_K1383002 BBa_K1383002], respectively). These RBS sequences were designed to have the theoretical greatest and worst possible affinities for the 16S ribosome. Figure 3 illustrates the specific sequence for the part described on this page, the 'native' RBS. | ||
[[Image:PMEV4-mCherry-Vector.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 1: The pMEV4-mCherry vector contains a region immediately upstream of the RFP, mCherry, labeled RBS 1-39. This site is where the native RBS and 38 variants thereof will be inserted.]] | [[Image:PMEV4-mCherry-Vector.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 1: The pMEV4-mCherry vector contains a region immediately upstream of the RFP, mCherry, labeled RBS 1-39. This site is where the native RBS and 38 variants thereof will be inserted.]] | ||
| − | [[Image:RBS-Variants.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 2: | + | [[Image:RBS-Variants.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 2: This list contains the sequences of the native RBS and the 38 variants thereof that make up the entire library.]] |
| − | [[Image:Native- | + | [[Image:Native-pmev4-mcherry-circuit.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 3: Specific sequence for the 'native' RBS. The figure is not drawn to scale.]] |
| + | |||
| + | |||
| + | |||
| + | '''2014: Improving the Characterization of Parts which include Fluorescent Reporters in Methanogens''' | ||
| + | |||
| + | Fluorescence readings in pAW50 ([https://parts.igem.org/Part:BBa_K1138002 BBa_K1138002]) were highly inconsistent and unreproducible. To remedy this, we discovered and addressed two main issues; 1) We removed resazurin from the broth-medium and 2) developed a novel protocol for oxygen exposure to mature the mCherry fluorophore. | ||
| + | |||
| + | Resazurin is an oxidation-reduction indicator which is commonly used in broth-mediums for obligate anaerobes because when resazurin is reduced, for example by oxygen, it turns a pink color which visually indicates to researchers that oxygen accidentally got into a culture. To run samples through a plate reader, we obviously had to take them out of their anaerobic culture and put them on a plate, which led to the reduction of resazurin and the false positives and large inconsistencies in characterization. This was a simple fix, since resazurin isn't required for growth, we made broth-medium that didn't contain resazurin and took extra caution to ensure no oxygen got into any cultures. | ||
| + | |||
| + | Many fluorescent proteins, including mCherry, require oxygen for the full maturation of the fluorophore. Since mCherry is being produced here in an obligate anaerobe, mCherry never had exposure to oxygen. We developed a novel protocol for oxygen exposure for maturation of the mCherry fluorophore produced in <i>M. maripaludis</i> cultures. This process, which may be found in full detail [http://2014.igem.org/Team:UGA-Georgia/Protocols here], briefly involves separating the cells, resuspending them in non-lysis buffer, and leave them in a shaker overnight. After around 20 hours of oxygen exposure, we observe visualization of mCherry (figure 4). | ||
| + | |||
| + | '''Qualitative Analysis''' | ||
| + | |||
| + | Since mCherry fluorescence is visible to the naked eye, visualization of mCherry after oxygen exposure is our qualitative analysis. The columns in figure 4 represent 37 'positive', native RBS (the part documented on this page), and 38 'negative', respectively. A close look would show that 37 'positive' looks the brightest, followed by 38 'negative', then the native RBS. Note on the right side of the picture a tube that contains wild-type <i>M. maripaludis</i> that also went through oxygen exposure. | ||
[[Image:MCherry-Visualization.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 4: Visualization of mCherry after 20h of oxygen exposure. The part described on this page, the native RBS, is shown in the middle column.]] | [[Image:MCherry-Visualization.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 4: Visualization of mCherry after 20h of oxygen exposure. The part described on this page, the native RBS, is shown in the middle column.]] | ||
| − | [[Image:MCherry-Quantitative.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 5: | + | '''Quantitative Analysis''' |
| + | |||
| + | A plate reader was used to evaluate quantitative values for the fluorescence of mCherry in <i>M. maripaludis</i> cultures. The graph in figure 5 depicts the values obtained from running our samples of 37 'positive', native RBS, and 38 'negative', which were derived from culture volumes of 5ml, 25ml, and 100ml. Triplicates of every sample were run and the values shown in the graph are the mean average of the triplicates. | ||
| + | |||
| + | [[Image:MCherry-Quantitative.png|none|500px|thumb|<b>UGA-Georgia 2014</b> Figure 5: This graph depicts the quantitative results of mCherry as read by a plate reader for the native RBS (documented on this page), the 37 'postive', and the 38 'negative' sequences.]] | ||
| + | |||
| + | '''2015: New Application of the Part''' | ||
| + | |||
| + | After development of this part along with BBa_K1383001 and BBa_K1383002, this part has been applied as a <b>standard</b> for our point mutation library. Rather than only being an expression tool for our developing library, it has become the sequence by which all our mutations are standardized against. This can be seen in Table 1 where the fluorescence values are all percentages of relative native expression. | ||
| + | |||
| + | |||
| + | [[Image:UGA-Georgia_Coverage_map_native.jpg|none|500px|]] | ||
| + | Figure 6: This graph depicts point mutation versus percent of native sequence fluorescence. The native's full sequence is depicted along the x-axis at 100% fluorescence. Not all values shown here have been made into parts, the only part shown is the G to T point mutation at the 4th base site (BBa_K1635000). | ||
| + | |||
| + | <b>Table 1: </b> This table shows the percent of native expression for 8 different mutations of the native sequence as well as the native sequence. This table includes both pre-existing parts and what will become future parts. | ||
| + | |||
| + | <https://static.igem.org/mediawiki/parts/1/1e/BBa_K1383000_UGA-Georgia.jpg> | ||
| − | |||
| − | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 22:11, 19 September 2015
BBa_K1383000 (mCherry- Native RBS)
Usage and Biology
The ribosome binding sites (RBS) of archaea are not well characterized. By creating and characterizing a library of RBS sequences, researchers will be able to express proteins of interest at variable levels of expression in Methanococcus maripaludis. Ribosome binding sites are typically 6-7 base pair sequences on a transcript that is complementary to the 3’ end of the 16S rRNA. After binding of the RBS to the ribosome, translation will be initiated. An RBS with higher affinity for the ribosome will result in higher rate of translation, and inversely, an RBS with lower affinity will result in lower rate of translation.
Characterization
Characterization of the RBS sequences was accomplished using the red fluorescent protein, mCherry as a reporter. Qualitative analysis was evaluated by visualization of the RFP and quantitative analysis was completed through use of a plate reader for reading fluorescence.
2013: Improving the Construct and Characterization of Fluorescent Reporters for use in Methanogens
In 2013, the UGA-Georgia team submitted the part BBa_K1138002, named pAW50-mCherry. This construct, designed for use as a fluorescent reporter in methanogens, had a few issues. First, the part was not 100% biobrick compatible due to an internal restriction site in the mCherry gene. The characterization of the part was also inconsistent among samples, and overall unreproducible. To improve upon the previous part, we designed pMEV4-mCherry (BBa_K1383000, figure 1). The primary differences between this construct and the pAW50-mCherry of 2013 is that pMEV4-mCherry does not contain the internal restriction site and the selective resistance gene against puromycin, our antibiotic for use in M. maripaludis, has an independent promoter. Therefore, pMEV4-mCherry is 100% biobrick compatible, and can more reliably function under increased selective pressure. Improvements on characterization are elaborated more below.
The region labeled 'RBS 1-39' in the pMEV4-mCherry vector (figure 1) is the site immediately upstream of the mCherry gene that will be subject to variation. Specifically, we will be mutating the 6 base pairs of the RBS, the 5 of the spacer, and the first base of the start codon. We begin with the 'native' RBS (BBa_K1383000, shown on this page), which is a known functional RBS sequence in methanogens and is typically used for creating synthetic parts. The RBS variants are created by making mutations on only one base at a time for every nucleotide different from the native (figure 2). We designed two additional RBS sequences based off the 16S rRNA data for M. maripaludis, termed theoretical 'perfect' and 'negative' (figure 2, #37 & #38, BBa_K1383001 & BBa_K1383002, respectively). These RBS sequences were designed to have the theoretical greatest and worst possible affinities for the 16S ribosome. Figure 3 illustrates the specific sequence for the part described on this page, the 'native' RBS.
2014: Improving the Characterization of Parts which include Fluorescent Reporters in Methanogens
Fluorescence readings in pAW50 (BBa_K1138002) were highly inconsistent and unreproducible. To remedy this, we discovered and addressed two main issues; 1) We removed resazurin from the broth-medium and 2) developed a novel protocol for oxygen exposure to mature the mCherry fluorophore.
Resazurin is an oxidation-reduction indicator which is commonly used in broth-mediums for obligate anaerobes because when resazurin is reduced, for example by oxygen, it turns a pink color which visually indicates to researchers that oxygen accidentally got into a culture. To run samples through a plate reader, we obviously had to take them out of their anaerobic culture and put them on a plate, which led to the reduction of resazurin and the false positives and large inconsistencies in characterization. This was a simple fix, since resazurin isn't required for growth, we made broth-medium that didn't contain resazurin and took extra caution to ensure no oxygen got into any cultures.
Many fluorescent proteins, including mCherry, require oxygen for the full maturation of the fluorophore. Since mCherry is being produced here in an obligate anaerobe, mCherry never had exposure to oxygen. We developed a novel protocol for oxygen exposure for maturation of the mCherry fluorophore produced in M. maripaludis cultures. This process, which may be found in full detail [http://2014.igem.org/Team:UGA-Georgia/Protocols here], briefly involves separating the cells, resuspending them in non-lysis buffer, and leave them in a shaker overnight. After around 20 hours of oxygen exposure, we observe visualization of mCherry (figure 4).
Qualitative Analysis
Since mCherry fluorescence is visible to the naked eye, visualization of mCherry after oxygen exposure is our qualitative analysis. The columns in figure 4 represent 37 'positive', native RBS (the part documented on this page), and 38 'negative', respectively. A close look would show that 37 'positive' looks the brightest, followed by 38 'negative', then the native RBS. Note on the right side of the picture a tube that contains wild-type M. maripaludis that also went through oxygen exposure.
Quantitative Analysis
A plate reader was used to evaluate quantitative values for the fluorescence of mCherry in M. maripaludis cultures. The graph in figure 5 depicts the values obtained from running our samples of 37 'positive', native RBS, and 38 'negative', which were derived from culture volumes of 5ml, 25ml, and 100ml. Triplicates of every sample were run and the values shown in the graph are the mean average of the triplicates.
2015: New Application of the Part
After development of this part along with BBa_K1383001 and BBa_K1383002, this part has been applied as a standard for our point mutation library. Rather than only being an expression tool for our developing library, it has become the sequence by which all our mutations are standardized against. This can be seen in Table 1 where the fluorescence values are all percentages of relative native expression.
Figure 6: This graph depicts point mutation versus percent of native sequence fluorescence. The native's full sequence is depicted along the x-axis at 100% fluorescence. Not all values shown here have been made into parts, the only part shown is the G to T point mutation at the 4th base site (BBa_K1635000).
Table 1: This table shows the percent of native expression for 8 different mutations of the native sequence as well as the native sequence. This table includes both pre-existing parts and what will become future parts.
< >
>
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]