Difference between revisions of "Part:BBa K1688008"
| Line 3: | Line 3: | ||
ModLac is a modified laccase (D439A/M510L CueO) with N-His6 tag attached to it via a linker sequence. | ModLac is a modified laccase (D439A/M510L CueO) with N-His6 tag attached to it via a linker sequence. | ||
| − | |||
| − | |||
| − | |||
<!-- --> | <!-- --> | ||
| Line 13: | Line 10: | ||
[[File:uppsala_modlacvsecol.png]] | [[File:uppsala_modlacvsecol.png]] | ||
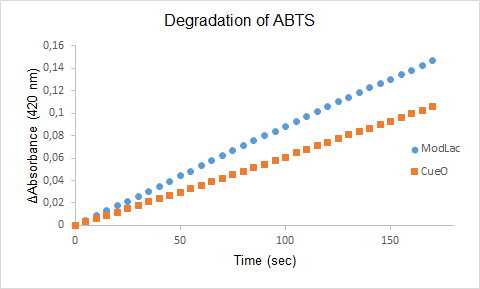
| − | + | Figure 1. The degradation of ABTS by the lysate of ModLac and the lysate of CueO over time. | |
| − | == | + | |
| − | + | ||
| − | + | == Usage and biology == | |
| + | |||
| + | |||
| + | Laccases (originally from Chinese lacquer tree sap) are multicopper oxidases, that are employed in various industries, where they take part in beer maturation, textile dyeing, and enzymatic biofuel cells. Due to their broad specificity and ability to oxidize aromatic compounds, their application in bioremediation is a topic under investigation. The laccase we chose is a modified laccase, CueO, a laccase from E. coli. The modified CueO laccase that we synthesized had a double mutation (D439A/M510L) that has been proven to increase the enzymatic activity. A polyhistidine-tag was also added at the N-terminus so that it could be purified easily. | ||
Revision as of 19:46, 18 September 2015
ModLac laccase with His-tag (inc RBS and J23110 promoter)
ModLac is a modified laccase (D439A/M510L CueO) with N-His6 tag attached to it via a linker sequence.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 310
- 1000COMPATIBLE WITH RFC[1000]
Figure 1. The degradation of ABTS by the lysate of ModLac and the lysate of CueO over time.
Usage and biology
Laccases (originally from Chinese lacquer tree sap) are multicopper oxidases, that are employed in various industries, where they take part in beer maturation, textile dyeing, and enzymatic biofuel cells. Due to their broad specificity and ability to oxidize aromatic compounds, their application in bioremediation is a topic under investigation. The laccase we chose is a modified laccase, CueO, a laccase from E. coli. The modified CueO laccase that we synthesized had a double mutation (D439A/M510L) that has been proven to increase the enzymatic activity. A polyhistidine-tag was also added at the N-terminus so that it could be purified easily.