Difference between revisions of "Part:BBa K1720002"
(→Experiment:) |
(→Experiment:) |
||
| Line 75: | Line 75: | ||
<b>Protocol:</b> | <b>Protocol:</b> | ||
1. Seed cells to be 40% confluent at a 35mm culture dish. | 1. Seed cells to be 40% confluent at a 35mm culture dish. | ||
| + | |||
2. Dilute 2.5ul Lipofectamine2000 Reagent in 50ul Opti-MEM Medium | 2. Dilute 2.5ul Lipofectamine2000 Reagent in 50ul Opti-MEM Medium | ||
| + | |||
3. Dilute 2.5ul (400ng/ul) plasmids in 50ul Opti-MEM Medium | 3. Dilute 2.5ul (400ng/ul) plasmids in 50ul Opti-MEM Medium | ||
| + | |||
4. Mix diluted Lipofectamine2000 Reagent with diluted plasmids, incubating for 5 min. | 4. Mix diluted Lipofectamine2000 Reagent with diluted plasmids, incubating for 5 min. | ||
| + | |||
5. Withdraw culture medium from 35mm culture dish. | 5. Withdraw culture medium from 35mm culture dish. | ||
| + | |||
6. Add 1ml Opti-MEM Medium and plasmid-Lipo complex to cells | 6. Add 1ml Opti-MEM Medium and plasmid-Lipo complex to cells | ||
| + | |||
7. Incubate for 15 hours. | 7. Incubate for 15 hours. | ||
| + | |||
8. Withdraw medium from culture dish. | 8. Withdraw medium from culture dish. | ||
| + | |||
9. Add 2ml DMEM medium( containing 10% FBS )to cells and incubate for 10 hours | 9. Add 2ml DMEM medium( containing 10% FBS )to cells and incubate for 10 hours | ||
| + | |||
10. Withdraw culture medium from 35mm culture dish. | 10. Withdraw culture medium from 35mm culture dish. | ||
| + | |||
11. Add 20ul sodium hyposulfite(100umol/L ) to cells | 11. Add 20ul sodium hyposulfite(100umol/L ) to cells | ||
| + | |||
12. Incubate for 1 hour. | 12. Incubate for 1 hour. | ||
| + | |||
13. Observe the cells under Inverted fluorescence microscope. | 13. Observe the cells under Inverted fluorescence microscope. | ||
| Line 93: | Line 105: | ||
<b>Result:</b> | <b>Result:</b> | ||
| − | + | [[File:SCUT2015_ChinaHRE-1_transfection.png|200px|thumb|left|Fig.2 Experimental Group]] | |
From the picture we can see that green fluorescence signal was only observed in experimental group and positive control, which indicated that this part work! | From the picture we can see that green fluorescence signal was only observed in experimental group and positive control, which indicated that this part work! | ||
Revision as of 00:48, 5 September 2015
Hypoxia-induced promotor
This part is a hypoxia response element.When the cells suffer from hypoxia situation this element will begin to work.It will activate the downstream gene expression.The hypoxia response element is a minimal cis-regulatory element mediating transactivation by the hypoxia-inducible factor (HIF) in mammalian cells.
This element with a GFP reporter was then transfected into HEK293 cells .Then we treat the cells with sodium hyposulfite, an reagent that leads to hypoxia. The negative control was HEK293 cells that treat with PBS and we did not detect green fluorescence signal.But we detected green fluorescence signal in the experimental group ,which meant that this element work under hypoxia situation.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal SpeI site found at 41
- 12INCOMPATIBLE WITH RFC[12]Illegal SpeI site found at 41
- 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal SpeI site found at 41
- 25INCOMPATIBLE WITH RFC[25]Illegal SpeI site found at 41
- 1000COMPATIBLE WITH RFC[1000]
Before we start the experiment, we designed an lentiviral vector that contains our part. and packaged the vector with lentivirus.
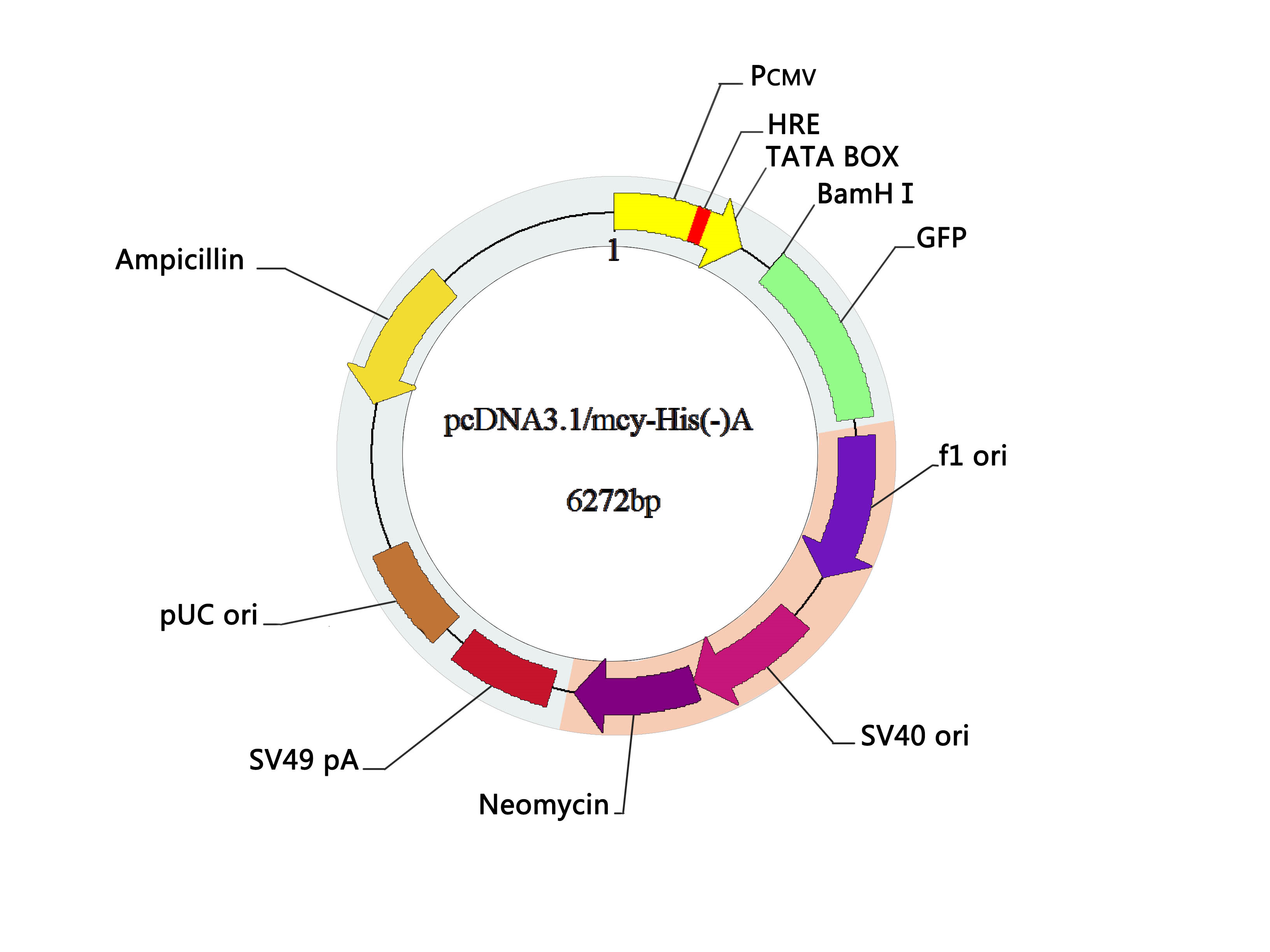
Vector Map:
Experiment:
We transiently transfected HEK293 cells with plasmids containing hypoxia-induced promotor and EGFP reporter. The cells were treated with sodium hyposulfite, an oxygen cleaner to cause hypoxia situation.If green fluorescence signal was observed only in experimental group and positive control, our Hypoxia-induced promotor’s function will be demonstrated.
Protocol:
1. Seed cells to be 40% confluent at a 35mm culture dish.
2. Dilute 2.5ul Lipofectamine2000 Reagent in 50ul Opti-MEM Medium
3. Dilute 2.5ul (400ng/ul) plasmids in 50ul Opti-MEM Medium
4. Mix diluted Lipofectamine2000 Reagent with diluted plasmids, incubating for 5 min.
5. Withdraw culture medium from 35mm culture dish.
6. Add 1ml Opti-MEM Medium and plasmid-Lipo complex to cells
7. Incubate for 15 hours.
8. Withdraw medium from culture dish.
9. Add 2ml DMEM medium( containing 10% FBS )to cells and incubate for 10 hours
10. Withdraw culture medium from 35mm culture dish.
11. Add 20ul sodium hyposulfite(100umol/L ) to cells
12. Incubate for 1 hour.
13. Observe the cells under Inverted fluorescence microscope.
Note:
The negative control was transiently transfected with the same plasmids in experimental group but did not treat with sodium hyposulfite. The positive control was transiently transfected with plasmids that contain CMV promoter and EGFP reporter.
Result:
From the picture we can see that green fluorescence signal was only observed in experimental group and positive control, which indicated that this part work!