Difference between revisions of "Part:BBa K1412716"
(→Protocol[1]) |
|||
| (12 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1412716 short</partinfo> | <partinfo>BBa_K1412716 short</partinfo> | ||
| − | This part | + | This part consists of Anderson promoter <bbpart>J23101</bbpart> and GFP generator <bbpart>BBa_E0240</bbpart>. It can be used for characterization of promoter <bbpart>J23101</bbpart>. When the device is constructed in backbone <bbpart>pSB3K3</bbpart> (<bbpart>BBa_K1412716</bbpart>). A low copy number is in expectation, as a result, a weak fluorescence strength is shown. While the device is constructed in <bbpart>pSB1C3</bbpart> (<bbpart>BBa_K1412924</bbpart>) which is a higher copy number vector, hence a stronger fluorescence strength, so that it can be obvious enough to be observed in naked eyes. |
=='''Usage and Biology'''== | =='''Usage and Biology'''== | ||
| − | + | The absolute activity of BioBrick promoters varies in experimental conditions and measurement instruments. We choose the promoter <bbpart>J23101</bbpart> as an in vivo reference standard for promoter activity. In order to get the different expression intensity of promoter, we connect the promoter with the same RBS, GFP generator and backbone. Finally we can use the intensity of fluorescent protein as a characterization data to report the expression intensity of different promoter. In order to get the different expression intensity of BioBrick backbones, we connect the backbones with the same promoter and GFP generator. Finally we can use the intensity of fluorescent protein as a characterization data to report the expression intensity of different backbones. | |
| + | |||
| + | =====<i>Relevent parts:</i>===== | ||
| + | |||
| + | =====<i><bbpart>BBa_K1412924</bbpart>: <bbpart>BBa_J23101</bbpart> + <bbpart>BBa_E0240</bbpart> (B0032-E0040-B0015), in the <bbpart>pSB1C3</bbpart> vector.</i>===== | ||
| + | |||
| + | =====<i><bbpart>BBa_K1412999</bbpart>: <bbpart>BBa_J23115</bbpart> + <bbpart>BBa_E0240</bbpart> (B0032-E0040-B0015), in the <bbpart>pSB1C3</bbpart> vector.</i>===== | ||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| Line 24: | Line 31: | ||
=='''Experimental data'''== | =='''Experimental data'''== | ||
| − | == | + | ==Verification== |
[[File:胶图验证1.png]] | [[File:胶图验证1.png]] | ||
| Line 32: | Line 39: | ||
1. 1kb Marker; | 1. 1kb Marker; | ||
| − | 2. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_J23115</bbpart> + <bbpart>BBa_E0240</bbpart> (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) | + | 2. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_J23115</bbpart> + <bbpart>BBa_E0240</bbpart> (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]); |
| − | 3. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_J23115</bbpart> + <bbpart>BBa_E0240</bbpart> (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) | + | 3. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_J23115</bbpart> + <bbpart>BBa_E0240</bbpart> (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]); |
| − | 4. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_I20260</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) | + | 4. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_I20260</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]); |
5. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_I20260</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]). | 5. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device <bbpart>BBa_I20260</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]). | ||
| Line 42: | Line 49: | ||
'''Results and discussion:''' | '''Results and discussion:''' | ||
| − | For device <bbpart>BBa_I20260</bbpart>, it’s very abnormal that we get puzzling results with double enzymes digestion by [http://en.wikipedia.org/wiki/Restriction_enzyme Xba I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]. The target segment seems vanished. However, if we use [http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I] instead, we find that the segments, whose backbone is <bbpart> | + | For device <bbpart>BBa_I20260</bbpart>, it’s very abnormal that we get puzzling results with double enzymes digestion by [http://en.wikipedia.org/wiki/Restriction_enzyme Xba I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]. The target segment seems vanished. However, if we use [http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I] instead, we find that the segments, whose backbone is <bbpart>PSB3K3</bbpart>, generated by mono-restriction digestion is about 1000 bp longer than that generated by double restriction enzyme digestion, and the devices have been verified by DNA sequencing. We can also get segments slightly shorter than 1000 bp which generated by double digestion, and those segments are highlighted by red frames. So we confirm that device <bbpart>BBa_I20260</bbpart> is correct. We took our actual measurement with the reconstructed device. |
---- | ---- | ||
| Line 50: | Line 57: | ||
====Figure 2. GFP of different device==== | ====Figure 2. GFP of different device==== | ||
| − | 1: | + | 1: <bbpart>BBa_K1412999</bbpart> in DH5α; |
| − | 2: | + | 2: <bbpart>BBa_K1412716</bbpart> in DH5α; |
| − | 3: | + | 3: <bbpart>BBa_K1412716</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) in DH5α; |
| − | 4: | + | 4: <bbpart>BBa_K1412924</bbpart> in DH5α. |
| − | + | The bacteria was cultured in the LB medium for 12 hrs at 37℃ shaking at 200 rpm in the table concentrator, then 1 ml bacterium solution was transferred into 1.5 ml centrifuge tube, and was centrifuged at 10000 rcf(g) for 1 min. The supernatant was discarded and the residuals was suspend by [http://en.wikipedia.org/wiki/Phosphate_buffered_saline PBS]. The solution was centrifuged again, and we got the bacteria precipitate as the picture shown in '''Figure 2'''. In which we can find that device <bbpart>BBa_K1412924</bbpart> is greenish in natural light while device <bbpart>BBa_K1412716</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) and <bbpart>BBa_K1412716</bbpart> emit a canary yellow color, and device <bbpart>BBa_K1412999</bbpart> show the color which is close to white. | |
---- | ---- | ||
| Line 66: | Line 73: | ||
====Figure 3. GFP of different device under the UV-light==== | ====Figure 3. GFP of different device under the UV-light==== | ||
| − | 1: | + | 1: <bbpart>BBa_K1412924</bbpart> in DH5α; |
| − | 2: | + | 2: <bbpart>BBa_K1412716</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) in DH5α; |
| − | 3: | + | 3: <bbpart>BBa_K1412716</bbpart> in DH5α; |
| − | 4: | + | 4: <bbpart>BBa_K1412999</bbpart> in DH5α. |
| − | + | Under UV-light, the bacterium precipitate above can be observed clearly that device <bbpart>BBa_K1412924</bbpart> can emit strong green fluorescence, while devices <bbpart>BBa_K1412716</bbpart> (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) and <bbpart>BBa_K1412716</bbpart> have weaker green fluorescence, and the green fluorescence from device <bbpart>BBa_K1412999</bbpart> is the weakest so that we can’t even observe a green pixel. | |
==Measurement== | ==Measurement== | ||
| Line 81: | Line 88: | ||
====Figure 4. The plot of optical density versus time==== | ====Figure 4. The plot of optical density versus time==== | ||
| − | From the plot of optical density versus time, we can conclude that the growth rate of bacteria is lower with time. We measured the | + | From the plot of optical density versus time, we can conclude that the growth rate of bacteria is become lower with time. We measured the samples three times parallelly, and we can know that the reproducibility of the data is acceptable. When we compare it with <bbpart>BBa_K1412924</bbpart> and <bbpart>BBa_K1412999</bbpart>, we can get that their growth rate are almost equal. |
---- | ---- | ||
| Line 89: | Line 96: | ||
====Figure 5. The plot of RFUs versus time==== | ====Figure 5. The plot of RFUs versus time==== | ||
| − | From the plot of RFUs versus time, we can conclude that RFUs grow linearly with time. When we compare with <bbpart>BBa_K1412924</bbpart> and <bbpart>BBa_K1412999</bbpart>, we can get that the fluoresent expression intensity of <bbpart>BBa_K1412716</bbpart> is higher than <bbpart>BBa_K141299</bbpart> | + | From the plot of RFUs(relative fluorescent units) versus time, we can conclude that RFUs grow linearly with time. When we compare it with <bbpart>BBa_K1412924</bbpart> and <bbpart>BBa_K1412999</bbpart>, we can get that the fluoresent expression intensity of <bbpart>BBa_K1412716</bbpart> is higher than <bbpart>BBa_K141299</bbpart> whike lower than <bbpart>BBa_K1412924</bbpart>. |
---- | ---- | ||
| Line 97: | Line 104: | ||
====Figure .6 The plot of RFUs versus OD600==== | ====Figure .6 The plot of RFUs versus OD600==== | ||
| − | From the plot of RFUs versus OD600, we can conclude that RFUs grow linearly with OD600. Because the fluoresent protein expression of each bateria is contain, so when the concentration of bacteria increase, the fluoresent expression increase too. When we compare with <bbpart>BBa_K1412716</bbpart> and <bbpart>BBa_K1412999</bbpart>, we can get that the fluoresent expression intensity of <bbpart>BBa_K1412716</bbpart> is higher than <bbpart>BBa_K141299</bbpart> | + | From the plot of RFUs versus OD600, we can conclude that RFUs grow linearly with OD600. Because the fluoresent protein expression of each bateria is contain, so when the concentration of bacteria increase, the fluoresent expression increase too. When we compare it with <bbpart>BBa_K1412716</bbpart> and <bbpart>BBa_K1412999</bbpart>, we can get that the fluoresent expression intensity of <bbpart>BBa_K1412716</bbpart> is higher than <bbpart>BBa_K141299</bbpart> whike lower than <bbpart>BBa_K1412924</bbpart>. |
---- | ---- | ||
| Line 105: | Line 112: | ||
====Figure 7. The plot of RFUs/OD600 versus time==== | ====Figure 7. The plot of RFUs/OD600 versus time==== | ||
| − | From the plot of RFUs/OD600 versus time, we know the RFUs/OD600 is a representation of the fluoresent expression intensity of unit bacteria. So we can get that the fluoresent expression intensity of <bbpart>BBa_K1412716</bbpart> is higher than <bbpart>BBa_K141299</bbpart> | + | From the plot of RFUs/OD600 versus time, we know the RFUs/OD600 is a representation of the fluoresent expression intensity of unit bacteria. So we can get that the fluoresent expression intensity of <bbpart>BBa_K1412716</bbpart> is higher than <bbpart>BBa_K141299</bbpart> whike lower than <bbpart>BBa_K1412924</bbpart>. |
| − | =='''Protocol'''== | + | =='''Protocol'''<sup>[1]</sup>== |
1. Transformed <bbpart>BBa_K1412716</bbpart> into DH5α competent cells, coated plates, grown in incubator for 12 hrs at 37℃. | 1. Transformed <bbpart>BBa_K1412716</bbpart> into DH5α competent cells, coated plates, grown in incubator for 12 hrs at 37℃. | ||
Latest revision as of 00:04, 18 October 2014
GFP generator with J23101
This part consists of Anderson promoter J23101 and GFP generator BBa_E0240. It can be used for characterization of promoter J23101. When the device is constructed in backbone pSB3K3 (BBa_K1412716). A low copy number is in expectation, as a result, a weak fluorescence strength is shown. While the device is constructed in pSB1C3 (BBa_K1412924) which is a higher copy number vector, hence a stronger fluorescence strength, so that it can be obvious enough to be observed in naked eyes.
Usage and Biology
The absolute activity of BioBrick promoters varies in experimental conditions and measurement instruments. We choose the promoter J23101 as an in vivo reference standard for promoter activity. In order to get the different expression intensity of promoter, we connect the promoter with the same RBS, GFP generator and backbone. Finally we can use the intensity of fluorescent protein as a characterization data to report the expression intensity of different promoter. In order to get the different expression intensity of BioBrick backbones, we connect the backbones with the same promoter and GFP generator. Finally we can use the intensity of fluorescent protein as a characterization data to report the expression intensity of different backbones.
Relevent parts:
BBa_K1412924: BBa_J23101 + BBa_E0240 (B0032-E0040-B0015), in the pSB1C3 vector.
BBa_K1412999: BBa_J23115 + BBa_E0240 (B0032-E0040-B0015), in the pSB1C3 vector.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 706
Experimental data
Verification
Figure 1. Enzyme digestion verification of devices BBa_J23115 + BBa_E0240 and BBa_I20260
1. 1kb Marker;
2. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_J23115 + BBa_E0240 (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]);
3. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_J23115 + BBa_E0240 (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]);
4. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_I20260 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]);
5. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_I20260 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]).
Results and discussion:
For device BBa_I20260, it’s very abnormal that we get puzzling results with double enzymes digestion by [http://en.wikipedia.org/wiki/Restriction_enzyme Xba I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]. The target segment seems vanished. However, if we use [http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I] instead, we find that the segments, whose backbone is PSB3K3, generated by mono-restriction digestion is about 1000 bp longer than that generated by double restriction enzyme digestion, and the devices have been verified by DNA sequencing. We can also get segments slightly shorter than 1000 bp which generated by double digestion, and those segments are highlighted by red frames. So we confirm that device BBa_I20260 is correct. We took our actual measurement with the reconstructed device.
Figure 2. GFP of different device
1: BBa_K1412999 in DH5α;
2: BBa_K1412716 in DH5α;
3: BBa_K1412716 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) in DH5α;
4: BBa_K1412924 in DH5α.
The bacteria was cultured in the LB medium for 12 hrs at 37℃ shaking at 200 rpm in the table concentrator, then 1 ml bacterium solution was transferred into 1.5 ml centrifuge tube, and was centrifuged at 10000 rcf(g) for 1 min. The supernatant was discarded and the residuals was suspend by [http://en.wikipedia.org/wiki/Phosphate_buffered_saline PBS]. The solution was centrifuged again, and we got the bacteria precipitate as the picture shown in Figure 2. In which we can find that device BBa_K1412924 is greenish in natural light while device BBa_K1412716 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) and BBa_K1412716 emit a canary yellow color, and device BBa_K1412999 show the color which is close to white.
Figure 3. GFP of different device under the UV-light
1: BBa_K1412924 in DH5α;
2: BBa_K1412716 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) in DH5α;
3: BBa_K1412716 in DH5α;
4: BBa_K1412999 in DH5α.
Under UV-light, the bacterium precipitate above can be observed clearly that device BBa_K1412924 can emit strong green fluorescence, while devices BBa_K1412716 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]) and BBa_K1412716 have weaker green fluorescence, and the green fluorescence from device BBa_K1412999 is the weakest so that we can’t even observe a green pixel.
Measurement
Figure 4. The plot of optical density versus time
From the plot of optical density versus time, we can conclude that the growth rate of bacteria is become lower with time. We measured the samples three times parallelly, and we can know that the reproducibility of the data is acceptable. When we compare it with BBa_K1412924 and BBa_K1412999, we can get that their growth rate are almost equal.
Figure 5. The plot of RFUs versus time
From the plot of RFUs(relative fluorescent units) versus time, we can conclude that RFUs grow linearly with time. When we compare it with BBa_K1412924 and BBa_K1412999, we can get that the fluoresent expression intensity of BBa_K1412716 is higher than BBa_K141299 whike lower than BBa_K1412924.
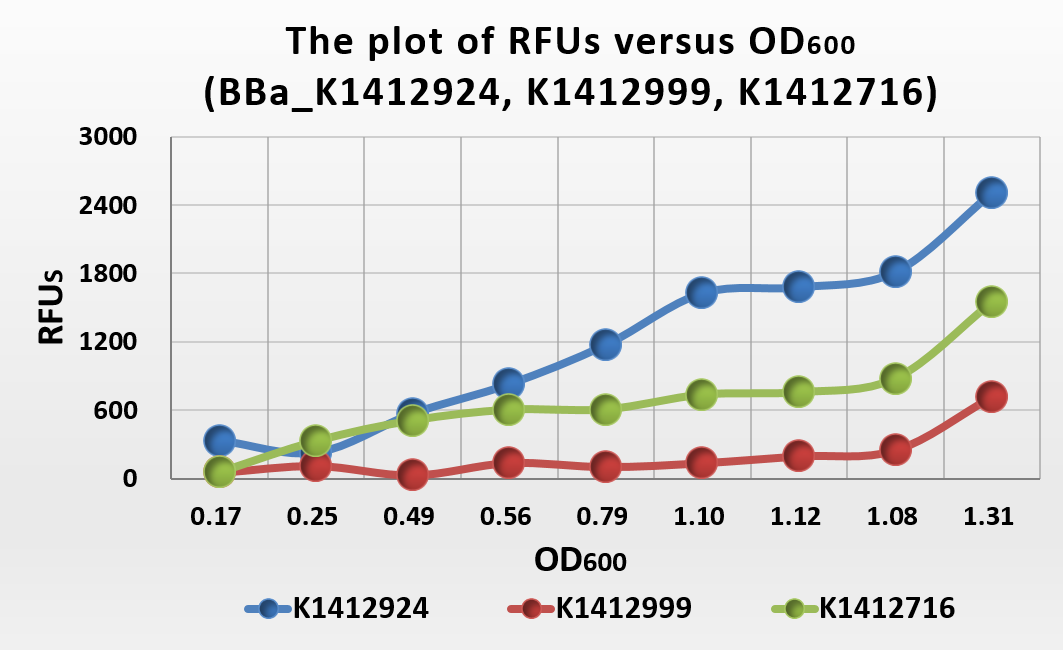
Figure .6 The plot of RFUs versus OD600
From the plot of RFUs versus OD600, we can conclude that RFUs grow linearly with OD600. Because the fluoresent protein expression of each bateria is contain, so when the concentration of bacteria increase, the fluoresent expression increase too. When we compare it with BBa_K1412716 and BBa_K1412999, we can get that the fluoresent expression intensity of BBa_K1412716 is higher than BBa_K141299 whike lower than BBa_K1412924.
Figure 7. The plot of RFUs/OD600 versus time
From the plot of RFUs/OD600 versus time, we know the RFUs/OD600 is a representation of the fluoresent expression intensity of unit bacteria. So we can get that the fluoresent expression intensity of BBa_K1412716 is higher than BBa_K141299 whike lower than BBa_K1412924.
Protocol[1]
1. Transformed BBa_K1412716 into DH5α competent cells, coated plates, grown in incubator for 12 hrs at 37℃.
2. Inoculate a 5 ml cultures of supplemented LB medium and antibiotic (Kanamycin 50 μg/ml) with single colony from the plate.
3. Cultures were grown in conical flask for 16 hrs at 37℃ with shaking at 200 rpm in the table concentrator.
4. Cultures were diluted 1:100 into three 20 ml fresh LB medium and grown for 3 hrs at 37℃ with shaking at 200 rpm in the table concentrator.
5. Then transfered 650 μl of the culture to a 1.5 ml centrifuge tube, centrifuged and washed twice with phosphate-buffered saline ([http://en.wikipedia.org/wiki/Phosphate_buffered_saline PBS], pH 7.4) to minimize the background fluorescence from the medium.
6. The washed cells were suspended in [http://en.wikipedia.org/wiki/Phosphate_buffered_saline PBS] and diluted to bring the cells into an appropriate concentration range (2–5 times) before taking fluorimeter measurements.
7. Measure the fluorescence and absorbance:
(1)Fluorescence:
- Device: [http://www.moleculardevices.com/systems/microplate-readers/multi-mode-readers/spectramax-m-series-multi-mode-microplate-readers SpectraMax+M5 microplate reader], 96-well plates.
- Wavelengths: 501 nm excitation, 514 nm emission, Auto-cutoff: 515 nm.
(2)OD600 (optical density at 600 nm):
- Device: [http://www.moleculardevices.com/systems/microplate-readers/multi-mode-readers/spectramax-m-series-multi-mode-microplate-readers SpectraMax+M5 microplate reader], 96-well plates.
- Wavelengths: 600 nm absorption.
8. Measure every 30 minutes in the next 4 hrs.
References
[1] [http://journals.aps.org/pre/abstract/10.1103/PhysRevE.82.021911 Bagh, Sangram, Mahuya Mandal, and David R. McMillen. "Minimal genetic device with multiple tunable functions." Physical Review E 82.2 (2010): 021911]
More information, click here: [http://2014.igem.org/Team:XMU-China# XMU-China]