Difference between revisions of "Part:BBa K1554001"
(→Usage and Biology) |
|||
| (4 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | The AtrΔ11 protein is a delta 11 desaturase coming from ''Amyelois transitella'' which introduces a desaturation in fatty acids at the 11th bond. | + | The AtrΔ11 protein is a delta-11-desaturase coming from ''Amyelois transitella'' which introduces a desaturation in fatty acids at the 11th bond. |
acyl-CoA + reduced acceptor + O2 = Delta11-acyl-CoA + acceptor + 2 H2O | acyl-CoA + reduced acceptor + O2 = Delta11-acyl-CoA + acceptor + 2 H2O | ||
| Line 14: | Line 14: | ||
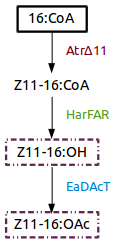
| − | [[File: | + | [[File:VUPV_pathway_parts.png]] |
Figure 1. Insect sexual pheromone pathway for ''Nicotiana benthamiana''. | Figure 1. Insect sexual pheromone pathway for ''Nicotiana benthamiana''. | ||
| + | |||
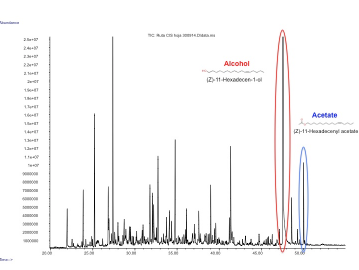
In our project we made a device with these three pheromones and expressed them by transient expression it in our plant chasis, ''Nicotiana benthamiana''. In order to check if the insect sexual pheromones were present, we performed the analysis using HS-SPME coupled to GC-MS. We observed two additional peaks in the transformed plants that were not present in the control and had a similar mass spectrum and retention time as the standards, which confirmed that both molecules were the desired pheromones, (Z)-11-hexadecen-1-ol and (Z)-11-hexadecenyl acetate. | In our project we made a device with these three pheromones and expressed them by transient expression it in our plant chasis, ''Nicotiana benthamiana''. In order to check if the insect sexual pheromones were present, we performed the analysis using HS-SPME coupled to GC-MS. We observed two additional peaks in the transformed plants that were not present in the control and had a similar mass spectrum and retention time as the standards, which confirmed that both molecules were the desired pheromones, (Z)-11-hexadecen-1-ol and (Z)-11-hexadecenyl acetate. | ||
| Line 27: | Line 28: | ||
Figure 3. GC-MS analysis of the volatile organic compounds from a genetically engineered ''Nicotiana benthamiana'' to produce insect pheromones. | Figure 3. GC-MS analysis of the volatile organic compounds from a genetically engineered ''Nicotiana benthamiana'' to produce insect pheromones. | ||
| + | |||
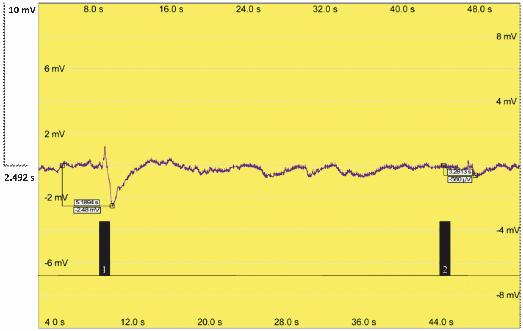
| + | Additionally, our team performed an electroantennography (EAG) to test the moth response to pheromones. We connected one antenna from a male moth, ''Sesamia nonagrioides'', with the two electrodes. Then, an air current with a leaf extract containing our pheromones was applied. As the extract was applied, the antenna transmitted an electrical impulse, meaning that there was response to our insect pheromones produced in plant. The volatiles in our plants induced detectable electric pulses that could indicate a pheromone response. | ||
| + | |||
| + | |||
| + | [[File:UPV_EAG.png]] | ||
| + | |||
| + | |||
| + | Figure 4. Electroantennography analysis of Sesamia nonagroides response to sexual pheromones produced in genetically engineered ''Nicotiana Benthamiana plants''. Signal 1: Antennal response to the Sexy Plant leaf extract. Signal 2: Antennal response to an air puff. | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Latest revision as of 17:39, 17 October 2014
AtrΔ11
The AtrΔ11 protein is a delta-11-desaturase from Amyelois transitella that introduces an unsaturation between C11 and C12 in long-chain fatty acids.
Usage and Biology
The AtrΔ11 protein is a delta-11-desaturase coming from Amyelois transitella which introduces a desaturation in fatty acids at the 11th bond.
acyl-CoA + reduced acceptor + O2 = Delta11-acyl-CoA + acceptor + 2 H2O
Part:BBa_K1554001 (AtrΔ11), Part:BBa_K1554002 (HarFAR) and Part:BBa_K1554003 (EaDAcT) are enzymes of a biosynthesis pathway that lead to the production of insect sexual pheromones, Z11-16:OH and Z11-16:OAc, using palmitate as substrate.
Figure 1. Insect sexual pheromone pathway for Nicotiana benthamiana.
In our project we made a device with these three pheromones and expressed them by transient expression it in our plant chasis, Nicotiana benthamiana. In order to check if the insect sexual pheromones were present, we performed the analysis using HS-SPME coupled to GC-MS. We observed two additional peaks in the transformed plants that were not present in the control and had a similar mass spectrum and retention time as the standards, which confirmed that both molecules were the desired pheromones, (Z)-11-hexadecen-1-ol and (Z)-11-hexadecenyl acetate.
Figure 2. GC-MS analysis of the volatile organic compounds from a negative control of Nicotiana benthamiana.
Figure 3. GC-MS analysis of the volatile organic compounds from a genetically engineered Nicotiana benthamiana to produce insect pheromones.
Additionally, our team performed an electroantennography (EAG) to test the moth response to pheromones. We connected one antenna from a male moth, Sesamia nonagrioides, with the two electrodes. Then, an air current with a leaf extract containing our pheromones was applied. As the extract was applied, the antenna transmitted an electrical impulse, meaning that there was response to our insect pheromones produced in plant. The volatiles in our plants induced detectable electric pulses that could indicate a pheromone response.
Figure 4. Electroantennography analysis of Sesamia nonagroides response to sexual pheromones produced in genetically engineered Nicotiana Benthamiana plants. Signal 1: Antennal response to the Sexy Plant leaf extract. Signal 2: Antennal response to an air puff.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]