Difference between revisions of "Part:BBa K1321306"
| Line 160: | Line 160: | ||
'''Natural antibiotics resistance of G.xylinus KI''' | '''Natural antibiotics resistance of G.xylinus KI''' | ||
| − | Using optimal antibiotics concentrations with G.xylinus KI is critical for selection of transformed cells while not inhibiting the cell growth. G.xylinus KI is naturally resistant to kanamycin, ampicillin and chloramphenicol concentrations normally used for E.coli (50µg/ml, 100µg/ml and 35µg/ml respectively; see Figure 4 for an experiment of KI natural antibiotic concentrations, experimental details, and comparison of ATCC and KI strains). The frequency of appearance of antibiotic-resistant colonies is dependent on the number of cells used for plating. Imperial iGEM 2014 team found that KI strain forms antibiotic-resistant colonies up to 6x antibiotic concentrations used for E.coli, thus the recommended amounts of antibiotics for transformations are 350µg/ml kanamycin, 700µg/ml ampicillin | + | Using optimal antibiotics concentrations with G.xylinus KI is critical for selection of transformed cells while not inhibiting the cell growth. G.xylinus KI is naturally resistant to kanamycin, ampicillin and chloramphenicol concentrations normally used for E.coli (50µg/ml, 100µg/ml and 35µg/ml respectively; see Figure 4 for an experiment of KI natural antibiotic concentrations, experimental details, and comparison of ATCC and KI strains). The frequency of appearance of antibiotic-resistant colonies is dependent on the number of cells used for plating - with higher number of plated cells, colonies appear even at higher antibiotics concentrations. Imperial iGEM 2014 team found that KI strain forms antibiotic-resistant colonies up to 6x antibiotic concentrations used for E.coli, thus the recommended amounts of antibiotics for HS-plates after transformations are 350µg/ml kanamycin, 700µg/ml ampicillin and 245µg/ml chloramphenicol. |
[[File:IC14 Antibiotics resistance experiment.jpg|400px|thumb|left| '''Figure 4.''' Natural antibiotics resistance of G.xylinus ATCC 53582 and KI strains. Due to small colony diameter and large clustering, colonies were rounded to the nearest hundred, if more than a hundred colonies were present. Plates with over 500 colonies often formed a lawn. Plates were seeded with 50µl of ATCC and KI seed culture normalized to equal OD600, and grown inverted at 30degC for 5 days until colony counting. N=3, error bars denote SD.]] | [[File:IC14 Antibiotics resistance experiment.jpg|400px|thumb|left| '''Figure 4.''' Natural antibiotics resistance of G.xylinus ATCC 53582 and KI strains. Due to small colony diameter and large clustering, colonies were rounded to the nearest hundred, if more than a hundred colonies were present. Plates with over 500 colonies often formed a lawn. Plates were seeded with 50µl of ATCC and KI seed culture normalized to equal OD600, and grown inverted at 30degC for 5 days until colony counting. N=3, error bars denote SD.]] | ||
Revision as of 12:03, 17 October 2014
Komagataeibacter rhaeticus iGEM
Gluconacetobacter xylinus is a obligate aerobe Gram negative cellulose-producing bacterial species. Imperial iGEM 2014 team isolated a strain of G.xylinus (named G. xylinus KI for Kombucha Isolate) from the Kombucha tea seed culture (Kombucha tea seed culture is a mixture of yeast, G.xylinus and other organisms, and is used to make the increasingly popular Kombucha tea). G. xylinus KI produces cellulose readily on different carbon feedstocks. Although G. xylinus KI does not produce as high amounts of cellulose as G. xylinus ATCC 53582 (see cellulose productivity on different feedstocks), it is more amenable to genetic engineering and testing of genetic constructs.
G.xylinus KI genome sequence
G.xylinus strain KI genome was sequenced as a part of Imperial 2014 iGEM project. The genome is about 3.5Mbp in size, with a GC content of about 60%.
Culturing G. xylinus KI
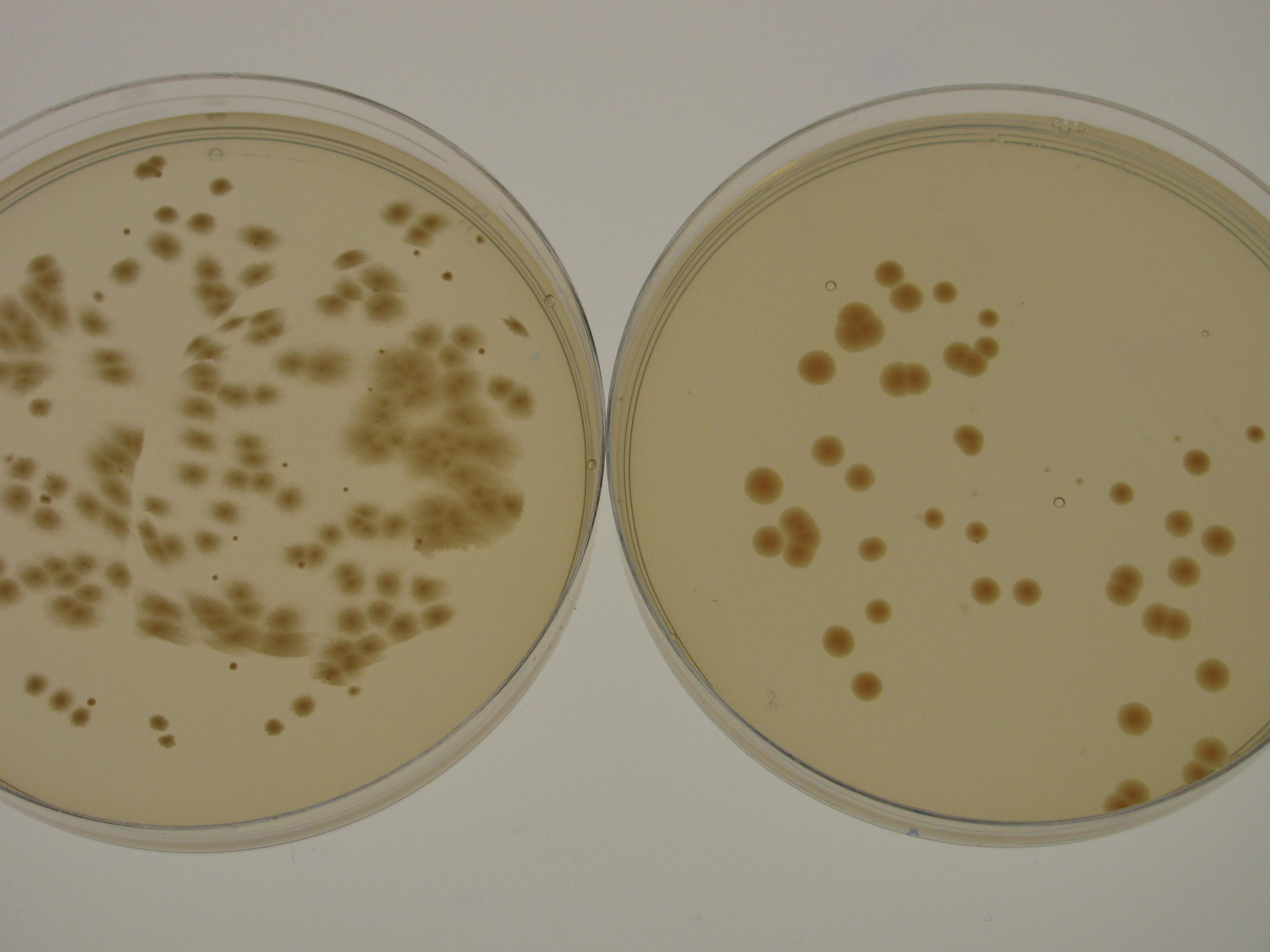
G.xylinus ATCC KI growth rate varies depending on the culturing conditions. Growth rate is highest when G.xylinus is cultured at 30degC, 180rpm shaking in a well-aerated setting. However, in shaking conditions, the prevalence of cellulose non-producing mutants (cel- mutants) increases, which causes a decrease in the total yield of produced cellulose. For this reason, when high cellulose production is required, G.xylinus is often grown in static conditions, at 30degC, in a well-aerated setting. In shaking cultures, KI strain reaches stationary phase in 48-72 hours, whereas in static cultures, depending on the volume of the medium, it can take up to 15 days. When grown on HS-agar plates at 30degC, cellulose-producing colonies become visible within 48-72 hours, cel- mutant colonies between 24 and 48 hours. Cellulose producing colonies can be distinguished from cel- mutants by colony morphology: cel+ have a rough colony morphology, whereas cel- mutants have a smooth colony morphology (see Figure 1).
The most commonly used medium for G.xylinum culturing is Hestrin-Schramm (HS) medium. HS medium contains the following components (for a full protocol for G.xylinus culturing, see G.xylinus culturing on [http://2014.igem.org/Team:Imperial/Protocols Imperial 2014 wiki]:
2% (w/v) glucose
0.5% (w/v) yeast extract
0.5% (w/v) peptone
0.27% (w/v) Na2HPO4
0.15% (w/v) citric acid.
Cellulose productivity of G.xylinus KI
G.xylinus KI cellulose productivity depends strongly on the carbon feedstocks used. In HS-glucose media grown for 7 days at 30degC standing using 50ml HS medium in 250ml conical flasks (sealed with foam buns), cellulose productivity is approximately one fifth of that of ATCC 53582 strain (see Figure 1 for comparison of ATCC53582 and KI strains and experimental details). However, KI growth is not optimal in HS-glucose, because in HS-glycerol media and HS-sucrose media the cellulose productivity is increased. Furthermore, in HS-sucrose medium, cellulose productivity is higher than that of ATCC 53582, which is of industrial significance due to the high availability of sucrose (see Feedstocks for G.xylinus KI).
Feedstocks for G.xylinus KI
G.xylinus is commonly grown on HS-glucose. However, it can readily use other carbon feedstocks, including sucrose and glycerol. have found that KI cellulose productivity is highest when grown on glycerol, and higher than ATCC 53582 when grown on sucrose (see Figure 2). Although glycerol seems to result in highest cellulose production, the effects of routine culturing of ATCC 53582 on HS-glycerol media are unknown.
Natural antibiotics resistance of G.xylinus KI
Using optimal antibiotics concentrations with G.xylinus KI is critical for selection of transformed cells while not inhibiting the cell growth. G.xylinus KI is naturally resistant to kanamycin, ampicillin and chloramphenicol concentrations normally used for E.coli (50µg/ml, 100µg/ml and 35µg/ml respectively; see Figure 4 for an experiment of KI natural antibiotic concentrations, experimental details, and comparison of ATCC and KI strains). The frequency of appearance of antibiotic-resistant colonies is dependent on the number of cells used for plating - with higher number of plated cells, colonies appear even at higher antibiotics concentrations. Imperial iGEM 2014 team found that KI strain forms antibiotic-resistant colonies up to 6x antibiotic concentrations used for E.coli, thus the recommended amounts of antibiotics for HS-plates after transformations are 350µg/ml kanamycin, 700µg/ml ampicillin and 245µg/ml chloramphenicol.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
