Difference between revisions of "Part:BBa K1060009"
(→Characterization of BBa_K1060009) |
|||
| (19 intermediate revisions by 3 users not shown) | |||
| Line 23: | Line 23: | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | ==Characterization of BBa_K1060009== | ||
| + | https://static.igem.org/mediawiki/2013/1/17/BBa_K1060009.jpg | ||
| + | |||
| + | ''E. coli'' BL21(DE3) strain | ||
| + | |||
| + | Mg2+: 5mM | ||
| + | |||
| + | control: BL21(DE3) without plasmid and antibiotics | ||
==System testing== | ==System testing== | ||
Our pilot experiment with aphid indicated that our brick performed the desired function, for the video of aphids experiment, please go to [http://www.youtube.com/watch?v=n-pMp8-XdUo Aphid Repellence video].<br/> | Our pilot experiment with aphid indicated that our brick performed the desired function, for the video of aphids experiment, please go to [http://www.youtube.com/watch?v=n-pMp8-XdUo Aphid Repellence video].<br/> | ||
| − | In this video you will find our first behavioural experiment with aphids. An EBF-producing bacterium plate (''E. coli'' BL21(DE3) on LB with 5mM Mg2+ and Chloramphenicol is placed on the left, a control plate on the right, aphids are placed on the leaf in the middle. The first results indicate a positive trend, as we can see them moving from left to right. (You may have to watch the video in full screen to clearly see the aphids.) For more information on the experimental setup and a discussion of the results go to [http://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/EBF#aphid%20experiments here]. | + | In this video you will find our first behavioural experiment with aphids. An EBF-producing bacterium plate (''E. coli'' BL21(DE3)) on LB with 5mM Mg2+ and Chloramphenicol is placed on the left, a control plate on the right, aphids are placed on the leaf in the middle. The first results indicate a positive trend, as we can see them moving from left to right. (You may have to watch the video in full screen to clearly see the aphids.) For more information on the experimental setup and a discussion of the results go to [http://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/EBF#aphid%20experiments here]. |
| − | For more | + | For more information see the experience page or go to [http://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/EBF#aphid%20experiments here]. |
| + | To back up our pilot experiment, quantitative analysis of aphid experiments was conducted. Three biological repeats for both a control setup (BL21 wild-type) and our EBF producing strain were used. Three infested leaves with similar size were placed in glass container for each setup, and the bacteria plates were only introduced 1 hour later (when the aphids were settled down on the leaves). The amount of aphids on top of the leaf as well as the amount of moving aphids were counted every 30 minutes. We observed a trend of more aphids scattering away from the leaves in the EBF setup but no statistical differences were seen in the control group, which proved that aphids were more agitated in the EBF setup compared to the control. | ||
| + | [[File:Bio assay top.JPG|600px|center]] | ||
| + | [[File:Bio assay moving.JPG|600px|center]] | ||
==SDS-PAGE confirmation== | ==SDS-PAGE confirmation== | ||
| − | As another approach to prove our constructs (<partinfo>BBa_K1060009</partinfo>,<partinfo>BBa_K1060011 </partinfo> and <partinfo>BBa_K1060014 </partinfo>), we transformed our different EBF synthase bricks in an <i>E.coli</i> expression strain, grew these up under different temperatures, times and, if possible, IPTG induction levels. Bacterial pellets were harvested and proteins extracted.<br/> | + | As another approach to prove our constructs (<partinfo>BBa_K1060009</partinfo>, <partinfo>BBa_K1060011 </partinfo> and <partinfo>BBa_K1060014 </partinfo>), we transformed our different EBF synthase bricks in an <i>E.coli</i> expression strain, grew these up under different temperatures, times and, if possible, IPTG induction levels. Bacterial pellets were harvested and proteins extracted.<br/> |
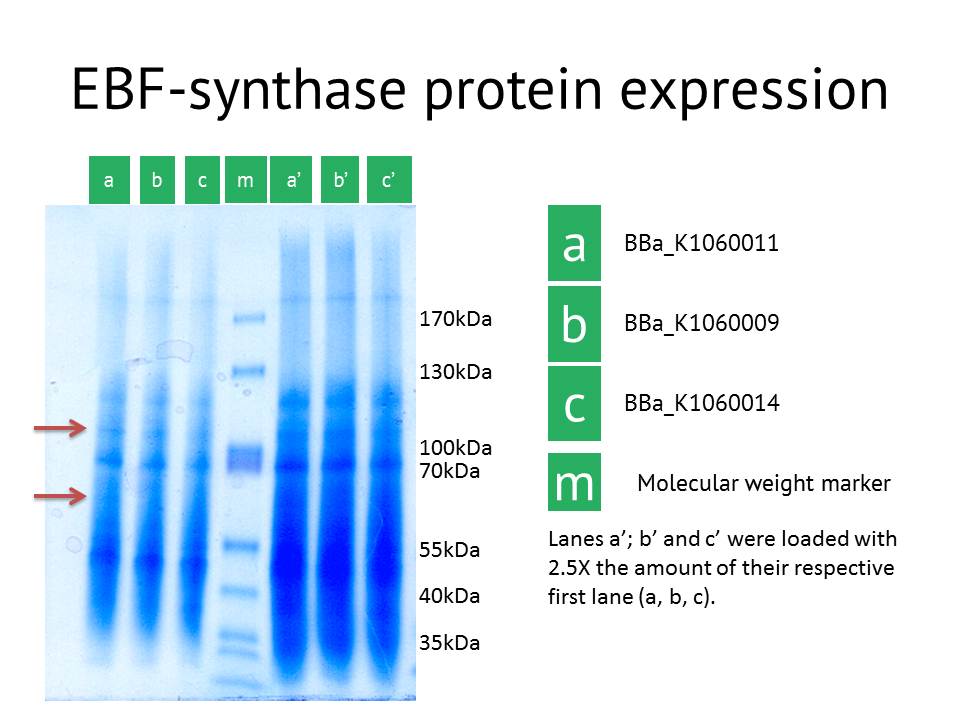
Here we showed the most interesting results. The figure shows some slight additional bands in lane a (around 110kDa and around 60 kDa), the protein extract from the lacI operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lane. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band. Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF synthase gene and possibly the increased production of a secondary protein. Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which would be equally inhibitory as a too low concentration. Other approaches to better identify the functionality of this construct would be via a gas chromatography analysis to directly measure the amounts of EBF produced. | Here we showed the most interesting results. The figure shows some slight additional bands in lane a (around 110kDa and around 60 kDa), the protein extract from the lacI operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lane. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band. Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF synthase gene and possibly the increased production of a secondary protein. Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which would be equally inhibitory as a too low concentration. Other approaches to better identify the functionality of this construct would be via a gas chromatography analysis to directly measure the amounts of EBF produced. | ||
| − | [[File: | + | [[File:EBFS_protein.JPG|600px|center]] |
| + | |||
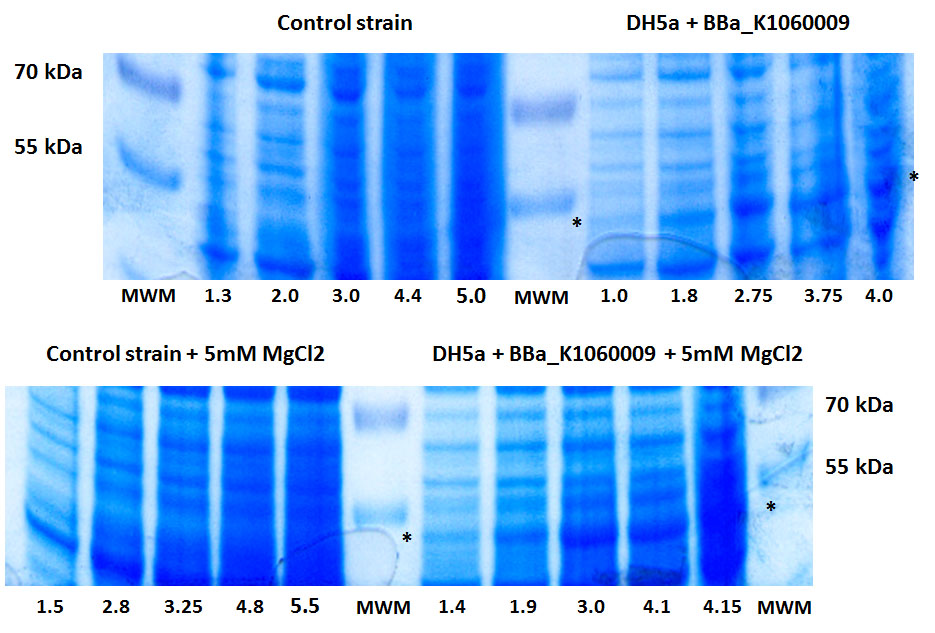
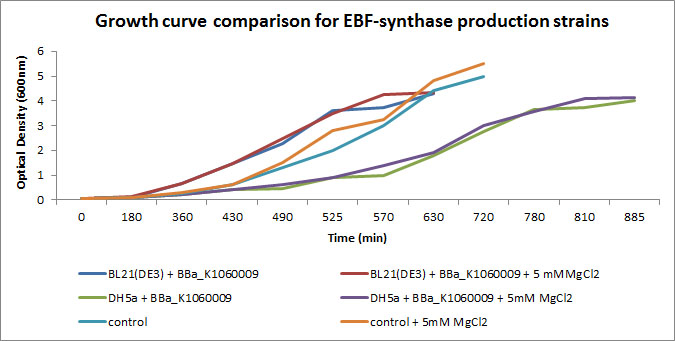
| + | In the figure below, we showed further characterizations: MgCl2 was added to the growth medium as it is suggested to improve solubility and functionality of EBF Synthase. We transformed BBa_K1060009 into DH5a and BL21(DE3), with/without MgCl2. Simultaneously we took an "empty" strain as a control, again with/without MgCl2. We grew overnight cultures of these 6 strains, inoculated 50ml each to a final Optical Density (600nm) of 0.05 and followed cellular growth over time (Growth curve figure). Simultaneously, we took samples for protein extraction at the OD600nm's indicated in SDS-PAGE figure. The growth curves showed that DH5a, transformed with BBaK1060009, had growth issues, irrespective of the presence of MgCl2. Interestingly, these cells showed an additional band around 50kDa (SDS-PAGE figure) which was not observed in the BL21(DE3) transformed strains (data not shown) nor the control strain (SDS-PAGE figure). Theoretical predictions suggest the EBF Synthase product should run around 66kDa, yet this still needs to be proven. (More details refer to [http://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/EBF here]) | ||
| + | |||
| + | [[File:Protein oct28.JPG|600px|center]] | ||
| + | |||
| + | [[File:Growthcurves EBFS.JPG|600px|center]] | ||
Latest revision as of 00:23, 29 October 2013
Medium constitutive expression of EBF synthase from Artemisia annua
For the production of the alarmhormone,(E)-β-farnesene, a sesquiterpene synthase gene (GenBank Accession No. AJ271793) was put behind a medium (constitutive) promoter and medium RBS (BBa_K608006).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30
Illegal NheI site found at 177 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1368
Illegal BamHI site found at 677
Illegal XhoI site found at 639 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 249
Construct digestion

Characterization of BBa_K1060009

E. coli BL21(DE3) strain
Mg2+: 5mM
control: BL21(DE3) without plasmid and antibiotics
System testing
Our pilot experiment with aphid indicated that our brick performed the desired function, for the video of aphids experiment, please go to [http://www.youtube.com/watch?v=n-pMp8-XdUo Aphid Repellence video].
In this video you will find our first behavioural experiment with aphids. An EBF-producing bacterium plate (E. coli BL21(DE3)) on LB with 5mM Mg2+ and Chloramphenicol is placed on the left, a control plate on the right, aphids are placed on the leaf in the middle. The first results indicate a positive trend, as we can see them moving from left to right. (You may have to watch the video in full screen to clearly see the aphids.) For more information on the experimental setup and a discussion of the results go to [http://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/EBF#aphid%20experiments here].
For more information see the experience page or go to [http://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/EBF#aphid%20experiments here].
To back up our pilot experiment, quantitative analysis of aphid experiments was conducted. Three biological repeats for both a control setup (BL21 wild-type) and our EBF producing strain were used. Three infested leaves with similar size were placed in glass container for each setup, and the bacteria plates were only introduced 1 hour later (when the aphids were settled down on the leaves). The amount of aphids on top of the leaf as well as the amount of moving aphids were counted every 30 minutes. We observed a trend of more aphids scattering away from the leaves in the EBF setup but no statistical differences were seen in the control group, which proved that aphids were more agitated in the EBF setup compared to the control.
SDS-PAGE confirmation
As another approach to prove our constructs (BBa_K1060009, BBa_K1060011 and BBa_K1060014), we transformed our different EBF synthase bricks in an E.coli expression strain, grew these up under different temperatures, times and, if possible, IPTG induction levels. Bacterial pellets were harvested and proteins extracted.
Here we showed the most interesting results. The figure shows some slight additional bands in lane a (around 110kDa and around 60 kDa), the protein extract from the lacI operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lane. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band. Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF synthase gene and possibly the increased production of a secondary protein. Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which would be equally inhibitory as a too low concentration. Other approaches to better identify the functionality of this construct would be via a gas chromatography analysis to directly measure the amounts of EBF produced.
In the figure below, we showed further characterizations: MgCl2 was added to the growth medium as it is suggested to improve solubility and functionality of EBF Synthase. We transformed BBa_K1060009 into DH5a and BL21(DE3), with/without MgCl2. Simultaneously we took an "empty" strain as a control, again with/without MgCl2. We grew overnight cultures of these 6 strains, inoculated 50ml each to a final Optical Density (600nm) of 0.05 and followed cellular growth over time (Growth curve figure). Simultaneously, we took samples for protein extraction at the OD600nm's indicated in SDS-PAGE figure. The growth curves showed that DH5a, transformed with BBaK1060009, had growth issues, irrespective of the presence of MgCl2. Interestingly, these cells showed an additional band around 50kDa (SDS-PAGE figure) which was not observed in the BL21(DE3) transformed strains (data not shown) nor the control strain (SDS-PAGE figure). Theoretical predictions suggest the EBF Synthase product should run around 66kDa, yet this still needs to be proven. (More details refer to [http://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/EBF here])