Difference between revisions of "Part:BBa K1060011"
(→SDS-PAGE confirmation) |
|||
| Line 18: | Line 18: | ||
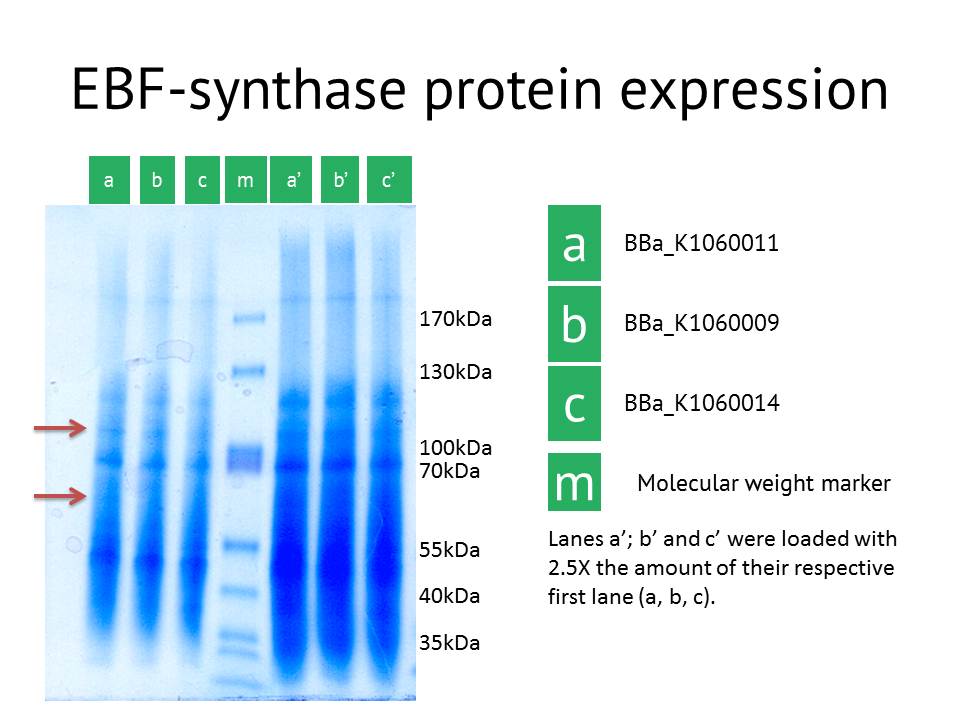
Here we showed the most interesting results. The figure shows some slight additional bands in lane a (around 110kDa and around 60 kDa), the protein extract from the lacI operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lane. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band. Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF synthase gene and possibly the increased production of a secondary protein. Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which would be equally inhibitory as a too low concentration. Other approaches to better identify the functionality of this construct would be via a gas chromatography analysis to directly measure the amounts of EBF produced. | Here we showed the most interesting results. The figure shows some slight additional bands in lane a (around 110kDa and around 60 kDa), the protein extract from the lacI operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lane. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band. Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF synthase gene and possibly the increased production of a secondary protein. Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which would be equally inhibitory as a too low concentration. Other approaches to better identify the functionality of this construct would be via a gas chromatography analysis to directly measure the amounts of EBF produced. | ||
| − | + | [[File:EBFS_protein.JPG|600px|center]] | |
Revision as of 01:25, 5 October 2013
Beta Farnesene production
For the production of the alarmhormone, (E)-β-farnesene, a sesquiterpene synthase gene (GenBank Accession No. AJ271793) was put behind a medium (constitutive) promoter and medium RBS (BBa_K608006). Additionally a Lac operator sequence (Gilbert & Maxam, 1973) was introduced to regulate its expression.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30
Illegal NheI site found at 209 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1409
Illegal BamHI site found at 709
Illegal XhoI site found at 671 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 281

SDS-PAGE confirmation
As another approach to prove our constructs (BBa_K1060009,BBa_K1060011 and BBa_K1060014), we transformed our different EBF synthase bricks in an E.coli expression strain, grew these up under different temperatures, times and, if possible, IPTG induction levels. Bacterial pellets were harvested and proteins extracted.
Here we showed the most interesting results. The figure shows some slight additional bands in lane a (around 110kDa and around 60 kDa), the protein extract from the lacI operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lane. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band. Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF synthase gene and possibly the increased production of a secondary protein. Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which would be equally inhibitory as a too low concentration. Other approaches to better identify the functionality of this construct would be via a gas chromatography analysis to directly measure the amounts of EBF produced.