Difference between revisions of "Part:BBa K1051259"
| Line 7: | Line 7: | ||
<h3>Principle</h3> | <h3>Principle</h3> | ||
'''SsrA degradation tag'''. In <i>E</i>. <i>coli</i>, the adaptor SspB tethers ssrAtagged substrates to the ClpXP protease, causing a modest increase in their rate of degradation. Which means, a variation of the WT SsrA tag sequence will accelerate the degradation of proteins when fused to their C-terminal. Thus the degradation rates are dependent on concentration of proteases and binding mediators. In order to fuse degradation tags freely on the C-terminal of protein, we add TAATAA to the tail of M0052 to construnt this K1051208.<br /> | '''SsrA degradation tag'''. In <i>E</i>. <i>coli</i>, the adaptor SspB tethers ssrAtagged substrates to the ClpXP protease, causing a modest increase in their rate of degradation. Which means, a variation of the WT SsrA tag sequence will accelerate the degradation of proteins when fused to their C-terminal. Thus the degradation rates are dependent on concentration of proteases and binding mediators. In order to fuse degradation tags freely on the C-terminal of protein, we add TAATAA to the tail of M0052 to construnt this K1051208.<br /> | ||
| + | The K1051208 is the improvement of M0052.(See more about M0052 at https://parts.igem.org/Part:BBa_M0052) | ||
We constructed the measurement pathway K1051259(contains J04500, K1051000 and K1051208) to test the rates of degradation of tagged proteins. J04450 was used as positive control because of the same promoter and fluorescent protein. | We constructed the measurement pathway K1051259(contains J04500, K1051000 and K1051208) to test the rates of degradation of tagged proteins. J04450 was used as positive control because of the same promoter and fluorescent protein. | ||
<br /> | <br /> | ||
| Line 31: | Line 32: | ||
<br /> | <br /> | ||
The fluorescence intensity and degradation rate of <i>E</i>. <i>coli</i>. | The fluorescence intensity and degradation rate of <i>E</i>. <i>coli</i>. | ||
| − | https://static.igem.org/mediawiki/2013/ | + | https://static.igem.org/mediawiki/2013/thumb/a/ad/DT-half_life_and_DR.png/800px-DT-half_life_and_DR.png |
<br /> | <br /> | ||
The data we calculated from microscope and microfluidic mate very well. | The data we calculated from microscope and microfluidic mate very well. | ||
Revision as of 08:27, 3 October 2013
Construct the measurement pathway of K1051208.
Purpose
Construct the measurement pathway of K1051208.
Principle
SsrA degradation tag. In E. coli, the adaptor SspB tethers ssrAtagged substrates to the ClpXP protease, causing a modest increase in their rate of degradation. Which means, a variation of the WT SsrA tag sequence will accelerate the degradation of proteins when fused to their C-terminal. Thus the degradation rates are dependent on concentration of proteases and binding mediators. In order to fuse degradation tags freely on the C-terminal of protein, we add TAATAA to the tail of M0052 to construnt this K1051208.
The K1051208 is the improvement of M0052.(See more about M0052 at https://parts.igem.org/Part:BBa_M0052)
We constructed the measurement pathway K1051259(contains J04500, K1051000 and K1051208) to test the rates of degradation of tagged proteins. J04450 was used as positive control because of the same promoter and fluorescent protein.
Measurement
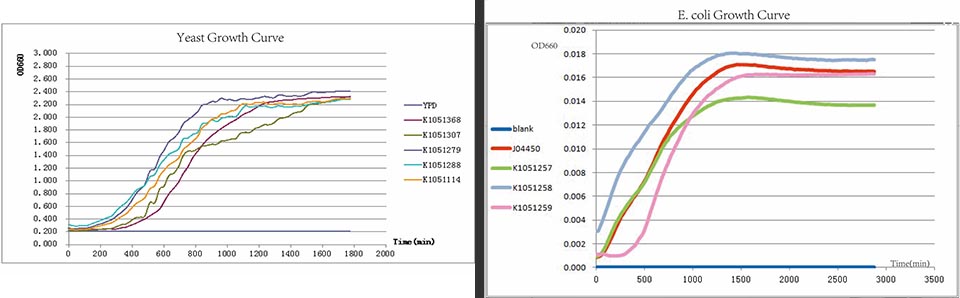 The growth curve of E. coli.
The growth curve of E. coli.
 From right to left, the negative control,BBa_K1051257, BBa_K1051258, BBa_K1051259, J04450 as Positive Control. As the pictures showed, the lights of RFP within three degradation tags are decreasing.
From right to left, the negative control,BBa_K1051257, BBa_K1051258, BBa_K1051259, J04450 as Positive Control. As the pictures showed, the lights of RFP within three degradation tags are decreasing.
 The test results of BBa_K1051258. A:No exciting lights; B. Powerful exciting lights; C. Weak exciting lights.
In picture, there are only obvious lights in the picture B, indicated the degradation rates are working
The test results of BBa_K1051258. A:No exciting lights; B. Powerful exciting lights; C. Weak exciting lights.
In picture, there are only obvious lights in the picture B, indicated the degradation rates are working
 The test results of BBa_K1051258 in chip. A,LB medium,O minuts; B, IPTG medium,9minutes; C,IPTG medium, 15 minutes
The test results of BBa_K1051258 in chip. A,LB medium,O minuts; B, IPTG medium,9minutes; C,IPTG medium, 15 minutes
 The average fluorescence intensity of K1051258 when added IPTG after specific time.
The average fluorescence intensity of K1051258 when added IPTG after specific time.

The fluorescence intensity and degradation rate of E. coli.

The data we calculated from microscope and microfluidic mate very well.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 781
Illegal AgeI site found at 893 - 1000COMPATIBLE WITH RFC[1000]
References
[1]McGinness, Baker, Sauer. 2006. Mol. Cell. 22:701.
[2]Flynn et al 2003. Mol. Cell. 11: 671. Flynn et al. 2001. PNAS 98(19): 10584. Anderson et al 1998. App. Env. Microbiol. 64(6):2240
