Difference between revisions of "Part:BBa K1119000"
(→Characterization) |
(→Characterization) |
||
| Line 21: | Line 21: | ||
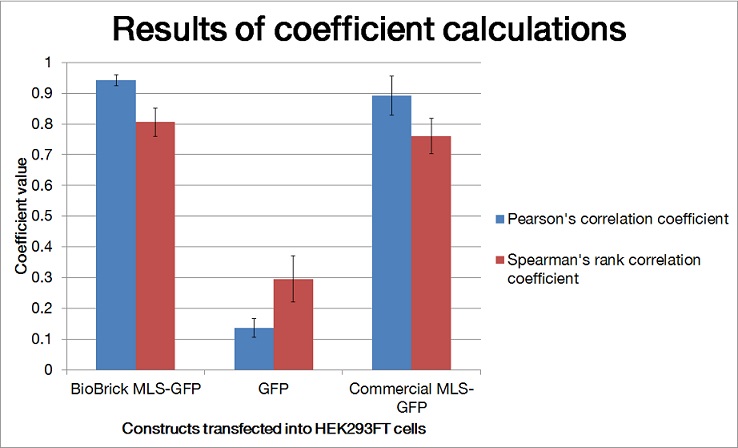
| − | [[File:scatterplots_mlsquantification.jpg|600px|thumb|center|'''Figure 2. Scatter plots of fluorescence intensities of green (y axis) and red (x axis) from images shown in Figure 1.''' Pearson's correlation coefficient (rp) and Spearman correlation coefficient (rs) were determined using the Pearson-Spearman correlation colocalization plugin (French et al., 2008) for ImageJ with a threshold of 0 and listed for each image.]] | + | [[File:scatterplots_mlsquantification.jpg|600px|thumb|center|'''Figure 2. Scatter plots of fluorescence intensities of green (y axis) and red (x axis) from images shown in Figure 1.'''It showed that the BioBrick MLS-GFP and commercial GFP construct had linear relationship of green intensities and red intensities while the GFP generator had no relationship. Pearson's correlation coefficient (rp) and Spearman correlation coefficient (rs) were determined using the Pearson-Spearman correlation colocalization plugin (French et al., 2008) for ImageJ with a threshold of 0 and listed for each image.]] |
Revision as of 10:33, 27 September 2013
Mitochondrial Leader Sequence in RFC10 standard
Mitochondrial Leader Sequence (MLS) helps direct protein to the mitochondria when this peptide sequence is in front of the N-terminus of the protein of interest. MLS will be removed upon the peptide’s translocation into the mitochondria, but four additional amino acid residues (Ile-His-Ser-Leu) will be left at the N-terminus of the protein. The CDS of MLS was cloned out from pCMV/myc/mito (Invitrogen, Carlsbard, CA) using PCR.
This part is in RFC10 standard but cannot be fused directly to other CDS due to limitations in RFC10. Users who obtained this part can extract the part by PCR and fuse to other domains using Splicing by Overlapping PCR.
MLS in RFC25 standard (BBa_K1119001) is also submitted to facilitate fusing with other CDS.
Characterization
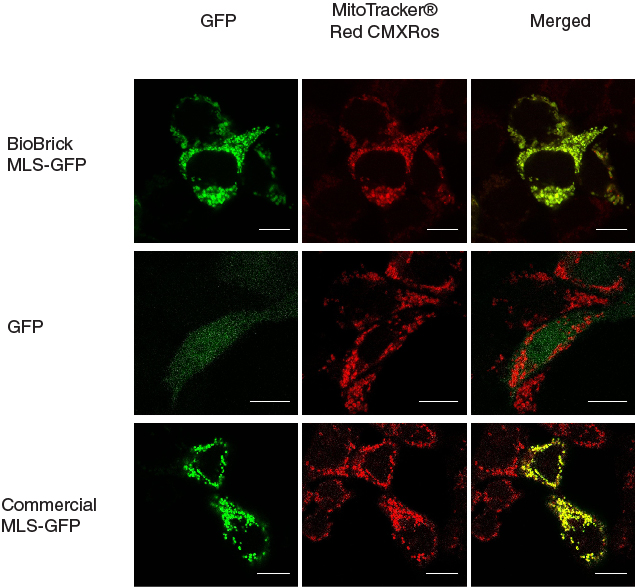
In our characterization, the CDS of MLS was assembled in frame with that of GFP reporter using Freiburg’s RFC25 format(BBa_K648013). The translation unit was driven by CMV promoter (BBa_K1119006) and terminated by hGH polyA signal (BBa_K404108).
The MLS-GFP generator (BBa_K1119009) was then transfected into HEK293FT cells. Mitochondria were stained after transfection and co-localization was determined by area of signal that overlapped.
To provide a positive control, CDS of EGFP from pEGFP-N1 (Clontech) was inserted downstream and in frame with the CDS of the MLS in the commercial plasmid pCMV/myc/mito, (Invitrogen, Carlsbard, CA). A negative control was made by GFP generator that does not contains the CDS of MLS (BBa_K1119008).
The [http://2013.igem.org/Team:Hong_Kong_HKUST/characterization detailed protocol] of our characterization can be found in HKUST iGEM 2013 Wiki.

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
