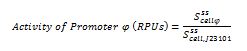Difference between revisions of "Part:BBa J23104:Experience"
Ao.patricia (Talk | contribs) (→User Reviews) |
|||
| Line 46: | Line 46: | ||
|} | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <I>'''iGEM CINVESTAV_IPN_UNAM''' CHARACTERIZATION OF IGEM DISTRIBUTION BIOPARTS</I> | ||
| + | |||
| + | For contribute to the parts registry our team decided to make the characterization of constitutive promoters belonging to the family isolated from a small combinatorial library (J23101 , J23102, J23104, J23107, J23108, J2311, and J23115) which were attached to GFP to determine promoter activity, using the equipment Victor X3 Multilabel Plate Reader. | ||
| + | |||
| + | [[Image:Gfp1.jpg]] | ||
| + | |||
| + | '''Fig. 1 Construction of the promoter J23102 expressing GFP.''' | ||
| + | |||
| + | '''Methods''' | ||
| + | |||
| + | With the selected colonies, an overnight culture was made in M9 media(minimal media supplemented with 0.2% CAA). After 12 hours the culture was transferred to a 96 well plate at a 1:10 dilution (20 μl of culture and 180 μL of fresh M9 medium). | ||
| + | OD and fluorescence measurements of the selected colonies were performed at intervals of 30 minutes for 16 h. | ||
| + | From the results the PopS were calculated (polymerases per second). | ||
| + | |||
| + | '''Modeling''' | ||
| + | |||
| + | The ecuations used for calulated de promoter activity were based on (R. K. Jason et. al 2009). | ||
| + | |||
| + | [[Image:ecu4.jpg]] | ||
| + | |||
| + | [[Image:ecu5.jpg]] | ||
| + | |||
| + | '''Results''' | ||
| + | |||
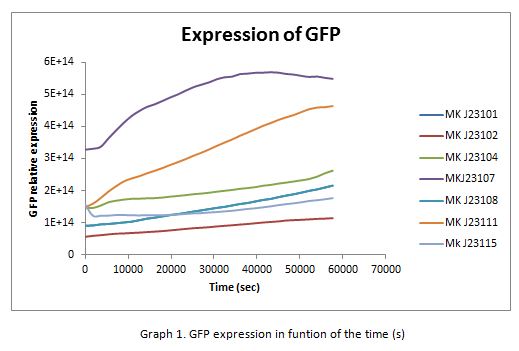
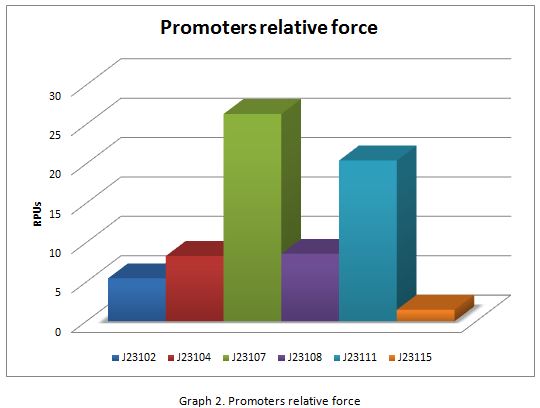
| + | In the following graphs there is shown the GFP expression in function of th time and the realtive promotor intensity. | ||
| + | |||
| + | |||
| + | [[Image:graf1.jpg]] | ||
| + | |||
| + | [[Image:graf2.jpg]] | ||
| + | |||
| + | |||
| + | With the previous results of the characterization of the promoters there is concluded that the promoter J23107, is the strongest because it produces more RPUs” | ||
Revision as of 18:38, 29 September 2012
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_J23104
User Reviews
UNIQ72d34fc079af7884-partinfo-00000000-QINU UNIQ72d34fc079af7884-partinfo-00000001-QINU
|
•••••
for iGEM-Team Goettingen 2012 |
Characterization experiment by qrtPCR on BBa_J23100, BBa_J23104, BBa_J23105, BBa_J23106, BBa_J23109, BBa_J23112, BBa_J23113, BBa_J23114 by iGEM Team Göttingen (by C. Krüger and J. Kampf)DescriptionWe used quantitative real-time PCR as a powerful tool for quantification of gene expression. We used this method to examine the expression rate of the Tar receptor gene under control of promoters from the Anderson family of the parts registry. The BioBricks (K777001-K777008) we used for this experiment can be found here. The reported activities of these promoters are given as the relative fluorescence of these plasmids in strain TG1 [1]. Promoter constructs were cloned into the vector pSB1C3 and expressed in E.coli BL21DE3 grown in LB-media (lysogeny broth). The measurements were performed for each construct and reference as a triplet. Additionally, we included H2O as negative control to predict possible contamination. For the evaluation of our results, the 2–ΔΔCT (Livak) method was applied. We used the weakest promoter with the lowest expression rate as calibrator for the calculations and as reference the housekeeping gene rrsD of E.coli.
You can find detailed information of the qrtPCR approach [http://2012.igem.org/Team:Goettingen/Project/Methods#-.3E_Experimental_design here].
Results & Discussion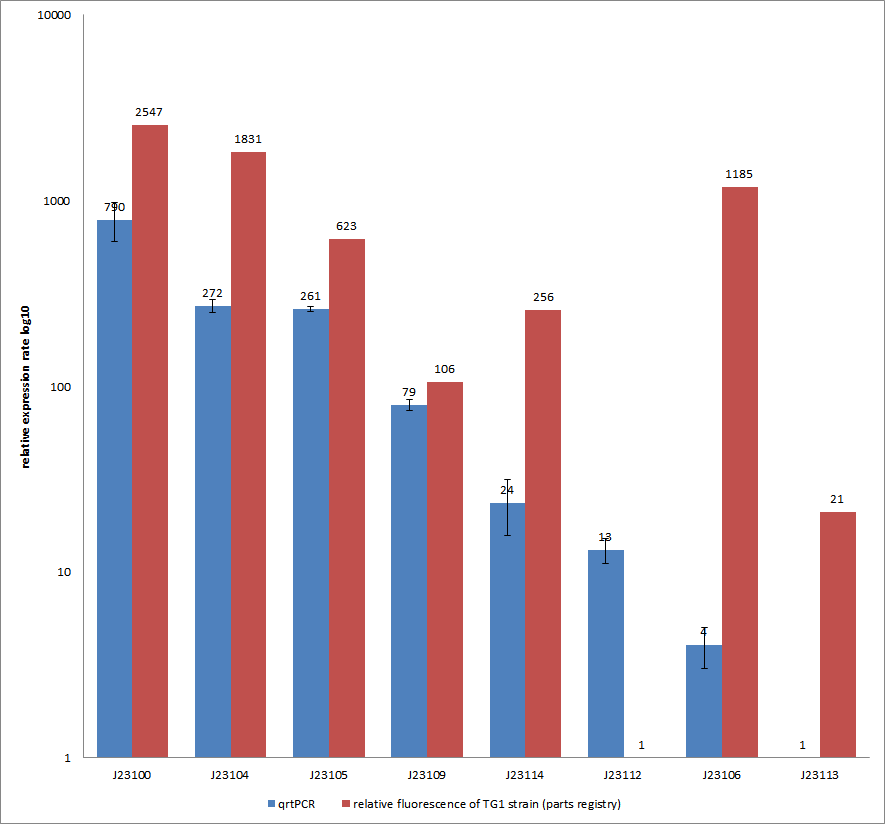 Comparison of relative expression rates of constitutive promoters by qrtPCR and relative fluorescence (see parts registry,Anderson family). The blue bar indicates the measured expression rates for our constructs (J23100, J23104, J23105, J23106, J23109, J23112, J23113, J23114) and the red ones those for the literature values represented in the “parts registry”. The measurements are illustrated in a logarithmic application. The standard variation was calculated for our measured values (black error bar). As mentioned before, both datasets were collected by methods which produce data at different points after the gene expression. Quantitative real time PCR measures the amount of expressed mRNA while relative fluorescence measurements quantify on protein level. In perspective of stability and half-life periods of mRNA and proteins or due to protein modification, it is comprehensible to obtain varying data-sets and expression rates. Another problem that occurred during our quantitative real-time measurements was the deviation in some of biological replicates. This problem was also observed in another group’s experiments ([http://www.jbioleng.org/content/3/1/4 Kelly et al., 2009]). They mentioned variations across experimental conditions in the absolute activity of the BioBricks. To reduce variation in promoter activity, they measured the activity of promoters relative to BBa_J23101. Furthermore, the iGEM team of Groningen which participated in 2009 also measured the relative fluorescence of TG1 strain with the promoters J23100, J23109 and J23106 via Relative Promoter Units (RPUs). Their values indicated the comparable tendency to our documented values |
iGEM CINVESTAV_IPN_UNAM CHARACTERIZATION OF IGEM DISTRIBUTION BIOPARTS
For contribute to the parts registry our team decided to make the characterization of constitutive promoters belonging to the family isolated from a small combinatorial library (J23101 , J23102, J23104, J23107, J23108, J2311, and J23115) which were attached to GFP to determine promoter activity, using the equipment Victor X3 Multilabel Plate Reader.
Fig. 1 Construction of the promoter J23102 expressing GFP.
Methods
With the selected colonies, an overnight culture was made in M9 media(minimal media supplemented with 0.2% CAA). After 12 hours the culture was transferred to a 96 well plate at a 1:10 dilution (20 μl of culture and 180 μL of fresh M9 medium). OD and fluorescence measurements of the selected colonies were performed at intervals of 30 minutes for 16 h. From the results the PopS were calculated (polymerases per second).
Modeling
The ecuations used for calulated de promoter activity were based on (R. K. Jason et. al 2009).
Results
In the following graphs there is shown the GFP expression in function of th time and the realtive promotor intensity.
With the previous results of the characterization of the promoters there is concluded that the promoter J23107, is the strongest because it produces more RPUs”