Difference between revisions of "Part:BBa K117000:Experience"
(→Applications of BBa_K117000) |
|||
| Line 11: | Line 11: | ||
For more detail description of our the Lysis part was used by the NTU@iGEM team, please visit: [http://2008.igem.org/Team:NTU-Singapore NTU@iGEM Team Main Page]. | For more detail description of our the Lysis part was used by the NTU@iGEM team, please visit: [http://2008.igem.org/Team:NTU-Singapore NTU@iGEM Team Main Page]. | ||
| + | |||
| + | == Application 2 == | ||
| + | we seu_a used and improved this part,details are as follow: | ||
| + | This part we use R0010 promoter ,it is to say in the absence of LacI protein and CAP protein, it promotes transcription,in the presence of LacI protein and CAP protein, it inhibits transcription. When induced by IPTG, the protein expression begin. | ||
| + | [[Image:seulfig8.jpg]] | ||
| + | Experimental procedure: | ||
| + | Step 1: Recovery J04500+K117000+B0015,shaking it in 37℃ for 12 to 16h at 150rpm. | ||
| + | Step 2: Measure the OD600 of bacterial liquid. | ||
| + | Step 3: Use 50ul bacterial liquid which has been diluted times to coat plates.Set IPTG(none or 1mM) control. | ||
| + | Step 4: Add 5ul bacterial liquid which has been diluted times to 5ml liquid Luria-Bertani medium. Set IPTG(none or 1mM) control. After cultivate in 37℃ shaking(150rmp) for 12 to 16h,measure the OD600 of bacterial liquid . | ||
| + | Step 5: Use 50ul bacterial liquid(from step 4) which has been diluted times to coat plates. Set IPTG(none or 1mM) control. | ||
| + | Step 6: Compare bacterial liquid concentration and petri dishes on the growth of the colony to judge lethal gene’s effects .Compare the bacterial concentration and Petri dish colony growth to judge the effect of lethal gene. Judging from the OD600 of bacterial liquid and the growth state of bacteria in Petri dish to see whether the death gene work or not. | ||
| + | [[Image:seulfig6.jpg]] | ||
| + | Fig 1. The experimental procedure of Death gene’s confirmatory experiment | ||
| + | |||
| + | The data of Sweet gene’s confirmatory experiment version 1.0 | ||
| + | |||
| + | |||
| + | |||
| + | Fig 2. The growth state of bacteria in Petri dish | ||
| + | (Notes: d/e/f means the bacterial liquid were diluted 104/105/106times,A/B means the concentration of IPTG none/1mM ) | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Fig 3. .The comparation of colony growth | ||
| + | |||
| + | (Notes: The left has IPTG of 1mM and the right has none) | ||
| + | |||
| + | |||
| + | |||
| + | |||
===User Reviews=== | ===User Reviews=== | ||
Revision as of 19:34, 26 September 2012
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K117000
This Biobrick part is a Lysis gene. It can be ligated to the desired choice of promoter and lysis of the E coli cells could be acheieved.
The NTU@iGEM did just that, and ligated this biobrick part to the pLsrA promoter which detect AI2, an auto-inducer molecule. After the pLsrA detects the AI2, the promoter promotes the transcripton of the lysis gene. Next translation takes place and the lysis protein is formed.
Lysis protein would lyse the cell by the destruction of the cell membrane.
For more detail description of our the Lysis part was used by the NTU@iGEM team, please visit: [http://2008.igem.org/Team:NTU-Singapore NTU@iGEM Team Main Page].
Application 2
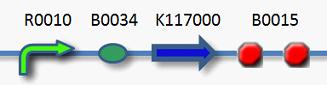
we seu_a used and improved this part,details are as follow: This part we use R0010 promoter ,it is to say in the absence of LacI protein and CAP protein, it promotes transcription,in the presence of LacI protein and CAP protein, it inhibits transcription. When induced by IPTG, the protein expression begin.
Experimental procedure: Step 1: Recovery J04500+K117000+B0015,shaking it in 37℃ for 12 to 16h at 150rpm. Step 2: Measure the OD600 of bacterial liquid. Step 3: Use 50ul bacterial liquid which has been diluted times to coat plates.Set IPTG(none or 1mM) control. Step 4: Add 5ul bacterial liquid which has been diluted times to 5ml liquid Luria-Bertani medium. Set IPTG(none or 1mM) control. After cultivate in 37℃ shaking(150rmp) for 12 to 16h,measure the OD600 of bacterial liquid . Step 5: Use 50ul bacterial liquid(from step 4) which has been diluted times to coat plates. Set IPTG(none or 1mM) control. Step 6: Compare bacterial liquid concentration and petri dishes on the growth of the colony to judge lethal gene’s effects .Compare the bacterial concentration and Petri dish colony growth to judge the effect of lethal gene. Judging from the OD600 of bacterial liquid and the growth state of bacteria in Petri dish to see whether the death gene work or not.
Fig 1. The experimental procedure of Death gene’s confirmatory experiment
The data of Sweet gene’s confirmatory experiment version 1.0
Fig 2. The growth state of bacteria in Petri dish (Notes: d/e/f means the bacterial liquid were diluted 104/105/106times,A/B means the concentration of IPTG none/1mM )
Fig 3. .The comparation of colony growth
(Notes: The left has IPTG of 1mM and the right has none)
User Reviews
UNIQac748af8bff8f4e6-partinfo-00000000-QINU UNIQac748af8bff8f4e6-partinfo-00000001-QINU