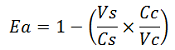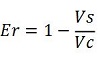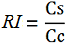Difference between revisions of "Part:BBa B0010:Experience"
| Line 35: | Line 35: | ||
<I>Trento iGEM team 2012</I> | <I>Trento iGEM team 2012</I> | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
| − | This part was successfully extracted from the distribution kits 2012 and 2011. However, it was not confirmed by restriction digestion nor PCR. We were able to amplify it by PCR from BBa_E0840, that contains the double terminator BBa_B0015, subcloned it into pSB1C3 and confirmed by sequencing. We re-deposited rrnBT1 terminator well characterized and functioning as [[Part:BBa_K731722|BBa_K731722]]. | + | This part was successfully extracted from the distribution kits 2012 and 2011. However, it was not confirmed by restriction digestion nor PCR. We were able to amplify it by PCR from BBa_E0840, that contains the double terminator BBa_B0015, subcloned it into pSB1C3 and confirmed by sequencing. We re-deposited rrnBT1 terminator well-characterized and well-functioning as [[Part:BBa_K731722|BBa_K731722]]. |
| + | |||
| + | |||
| + | The characterization of this part was done using the new platforms for terminator characterization that they have built and submitted to the Registry as [[Part:BBa_K731700|BBa_K731700]], [[Part:BBa_K731710|BBa_K731710]]. | ||
| + | |||
| + | Its activity was analyzed both with T7 and E. coli RNA polymerases in vivo and with T7 RNA polymerase in vitro. | ||
| + | |||
| + | The parameters used to analyze the data are: | ||
| + | |||
| + | apparent termination efficiency, [[Image:FormulaEa.jpg]] | ||
| + | |||
| + | raw termination efficiency, [[Image:FormulaEr.jpg]] | ||
| + | |||
| + | relative increase in the upstream gene expression, [[Image:RIequation.png]] | ||
| + | |||
| + | (the last parameter was added as some terminators were found to increase the expression of the upstream gene) | ||
| + | |||
| + | where | ||
| + | |||
| + | -Vs is the A206K Venus peak’s intensity of the construct with the terminator of interest inserted in the prefix-suffix linker | ||
| + | |||
| + | -Vc is the A206K Venus peak’s intensity of the control construct with no terminator | ||
| + | |||
| + | -Cs is the mCherry peak’s intensity of the construct with the terminator inserted | ||
| + | |||
| + | -Cc is the mCherry peak’s intensity of the control construct | ||
| + | |||
| + | |||
| + | |||
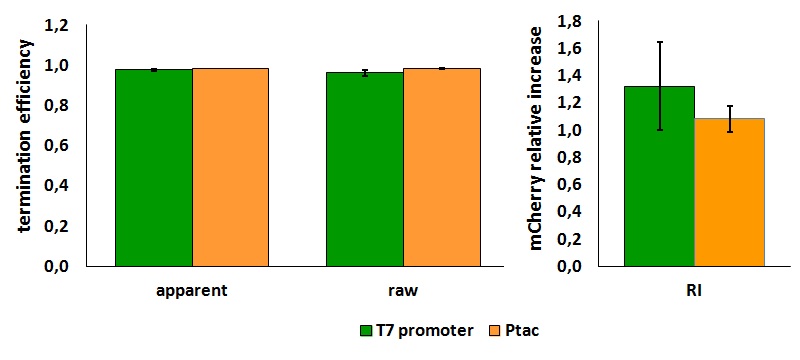
| + | '''In vivo measurements:''' | ||
| + | |||
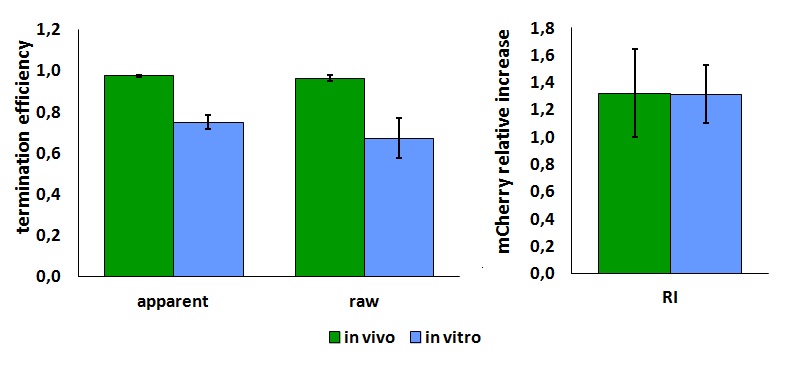
| + | <div style="text-align:center">[[Image:Ecoliterminatorinvivo.jpg]]</div> | ||
| + | |||
| + | |||
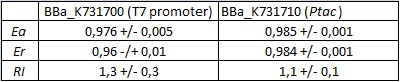
| + | <div style="text-align:center">[[Image:Ecoliterminatortabinvivo.jpg]]</div> | ||
| + | <p style="width:600px; margin-left:150px; margin-bottom:60px; | ||
| + | text-align:justify "><em><strong>FIGURE 1.</strong> '''E.coli terminator's effect on protein expression with two different RNA polymerases'''<br/>The data shown in figure 1 were acquired in two different days. For each day 4 different replicates were measured at different times. | ||
| + | |||
| + | Briefly, BL21(DE3)pLysS were grown in 10 mL of LB until an OD of 0.6 was reached and induced with 0.5 mM IPTG. After 3 hours of induction, 4 separate aliquots of 1 mL were taken and sonicated 3 times for 10 seconds at intervals of 30 seconds. After sonication the samples were diluted 1:3 with PBS 1X directly into a cuvette and incubated overnight at 4°C. Fluorescence measurements were taken with a Cary Eclipse Varian fluorimeter with a window ranging from 450 nm to 700 nm using the following excitation and emission wavelengths. | ||
| + | |||
| + | </em> </p> | ||
| + | |||
| + | |||
| + | |||
| + | '''In vitro measurements:''' | ||
| + | |||
| + | <div style="text-align:center">[[Image:Ecoliterminatorinvitro.jpg]]</div> | ||
| + | |||
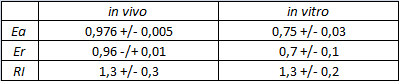
| + | <div style="text-align:center">[[Image:Ecoliterminatortabinvitro.jpg]]</div> | ||
| + | <p style="width:600px; margin-left:150px; margin-bottom:60px; | ||
| + | text-align:justify "><em><strong>FIGURE 2.</strong> '''E.coli terminator's effect on in vitro protein synthesis with the T7 RNA polymerases'''<br/>Cell free measurements were done with the PurExpress kit from New England Biolabs, using 250 ng of DNA previously purified by ethanol precipitation, following the protocol suggested by the manufacturer. Measurements were done with a PTI fluorimeter using the following excitation and emission wavelengths. | ||
| + | |||
| + | </em> </p> | ||
| + | |||
| + | More information can be found in the iGEM Trento 2012 wiki page. | ||
|}; | |}; | ||
<!-- End of the user review template --> | <!-- End of the user review template --> | ||
<!-- DON'T DELETE --><partinfo>BBa_B0010 EndReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_B0010 EndReviews</partinfo> | ||
Revision as of 16:45, 20 September 2012
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_B0010
- PCR Problems: Using primers VR and VF2 to PCR B0010 results in excess bands. VR can anneal to B0010, resulting in shorter bands than expected. A full description of the problem is available here.
User Reviews
UNIQ143cc1fb8f30dac2-partinfo-00000000-QINU
|
Antiquity |
This review comes from the old result system and indicates that this part did not work in some test. |
|
Trento iGEM team 2012 |
This part was successfully extracted from the distribution kits 2012 and 2011. However, it was not confirmed by restriction digestion nor PCR. We were able to amplify it by PCR from BBa_E0840, that contains the double terminator BBa_B0015, subcloned it into pSB1C3 and confirmed by sequencing. We re-deposited rrnBT1 terminator well-characterized and well-functioning as BBa_K731722.
Its activity was analyzed both with T7 and E. coli RNA polymerases in vivo and with T7 RNA polymerase in vitro. The parameters used to analyze the data are: apparent termination efficiency, relative increase in the upstream gene expression, (the last parameter was added as some terminators were found to increase the expression of the upstream gene) where -Vs is the A206K Venus peak’s intensity of the construct with the terminator of interest inserted in the prefix-suffix linker -Vc is the A206K Venus peak’s intensity of the control construct with no terminator -Cs is the mCherry peak’s intensity of the construct with the terminator inserted -Cc is the mCherry peak’s intensity of the control construct
In vivo measurements:
FIGURE 1. E.coli terminator's effect on protein expression with two different RNA polymerases
In vitro measurements: FIGURE 2. E.coli terminator's effect on in vitro protein synthesis with the T7 RNA polymerases More information can be found in the iGEM Trento 2012 wiki page. |
UNIQ143cc1fb8f30dac2-partinfo-00000003-QINU