Difference between revisions of "Part:BBa K658016"
| Line 6: | Line 6: | ||
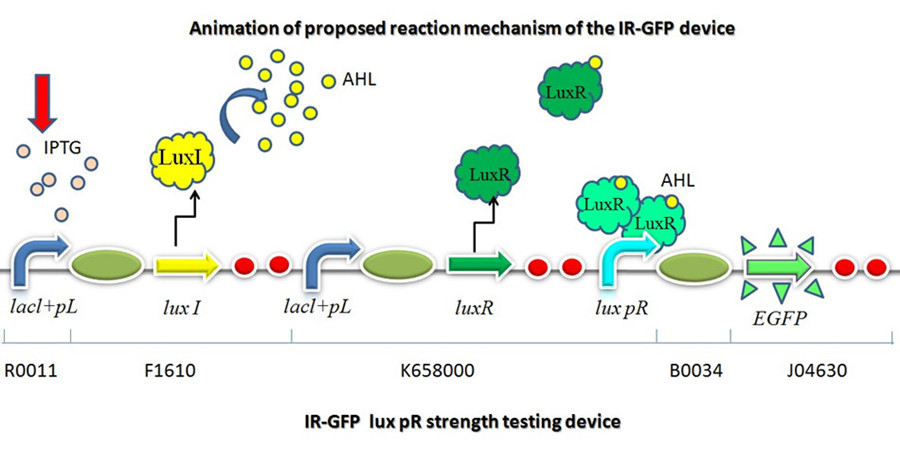
This part is designed to test the strength of promoters lux pR(BBa_R0062) and its 3 mutants lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007), lux pR-3/5(BBa_K658008) in our project. If promoter lacl+pL(BBa_R0011) is induced by isopropyl-b-D-thiogalactopyranoside (IPTG), this device will be switched on. At sufficiently high cell density, this device produces greenish tint visible by naked eye. By measuring florescent intensities at steady state of the cell growth for these four IR-GFP devices, the strength of a promoter lux pR could be defined. | This part is designed to test the strength of promoters lux pR(BBa_R0062) and its 3 mutants lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007), lux pR-3/5(BBa_K658008) in our project. If promoter lacl+pL(BBa_R0011) is induced by isopropyl-b-D-thiogalactopyranoside (IPTG), this device will be switched on. At sufficiently high cell density, this device produces greenish tint visible by naked eye. By measuring florescent intensities at steady state of the cell growth for these four IR-GFP devices, the strength of a promoter lux pR could be defined. | ||
| − | [[Image: | + | [[Image:XMU_China_1_forframe.jpg|Figure 1 animation of proposed reaction mechanism of IR-GFP device.|frame|Figure 1 animation of proposed reaction mechanism of IR-GFP device.]] |
[[Image:XMU_China_1.jpg|Figure 1 animation of proposed reaction mechanism of IR-GFP device.]] | [[Image:XMU_China_1.jpg|Figure 1 animation of proposed reaction mechanism of IR-GFP device.]] | ||
Figure 1 animation of proposed reaction mechanism of IR-GFP device. | Figure 1 animation of proposed reaction mechanism of IR-GFP device. | ||
Revision as of 04:05, 4 October 2011
LuxI->LuxR->lux pR->GFP
Lux pR strength testing device
This part is designed to test the strength of promoters lux pR(BBa_R0062) and its 3 mutants lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007), lux pR-3/5(BBa_K658008) in our project. If promoter lacl+pL(BBa_R0011) is induced by isopropyl-b-D-thiogalactopyranoside (IPTG), this device will be switched on. At sufficiently high cell density, this device produces greenish tint visible by naked eye. By measuring florescent intensities at steady state of the cell growth for these four IR-GFP devices, the strength of a promoter lux pR could be defined.
 Figure 1 animation of proposed reaction mechanism of IR-GFP device.
Figure 1 animation of proposed reaction mechanism of IR-GFP device.
Description
This device is made up of three subparts: a LuxI producer, a Lux R producer and a EGFP reporter. The luxI producer and luxR producer in this device work as a 3OC6HSL sender and receiver device driven by lacl+pL(R0011). We constructed the part which expresses LuxI and LuxR upon induction by isopropyl-b-D-thiogalactopyranoside (IPTG). There are two regulatory genes involved in quorum sensing, the I and R genes. The I gene directs the synthesis of an acyl-homoserine lactone (AHL) signal molecule termed the autoinducer. The R gene codes for a transcription factor that is responsive to the N-acyl HSL signal. Cells are permeable to this signal, and thus high cell densities are required to achieve a critical concentration of the autoinducer required to bind the luxR product, which will combine with lux pR and active the EGFP to transcribe.
Figure 2 XXXxxxxxxxxxxxxxxxxxxxx
Figure2: Two molecules of LuxR protein form a complex with two molecules of the signalling compound homoserine lactone (HSL). This complex binds to a palindromic site on the promoter, increasing the rate of transcription.
Figure 3 XXXxxxxxxxxxxxxxxxxxxxxxx
Figure 3: the sequence of promoter lux pR
On the basis of the nucleotide sequence of the lux pR promoter, the 20 base pair inverted repeat ACCTGTAGGA TCGTACAGGT might consititude a protein binding site. And we also learned that mutagenesis at position 3 and position 5 might cause dramatic change on the expression of downstream gene. Therefore, we first generated 3 mutants (BBa_K658006 BBa_K658007 BBa_K658008) of the promoter lux pR by site-directed mutagenesis, then we tested their strength in this IR-GFP device by measuring fluorescence at steady state.
Result
Four lux pR strength testing devices (BBa_K658016 BBa_K658017 BBa_K658018 BBa_K658019) were first cloned into plasmid pSB1A2 respectively, followed by transformation into E.coli strain BL21. Fluorescence was measured when cell growth reached a steady state (around 20h). The results are shown in following figures:
Figure 4 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
Figure 4: promoters strength relative to lux pR-3 (BBa_K658006)
FIGURE 5 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
Figure 5: efficiency of promoter lux pR and its three mutants
Figure 6 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
Figure 6: Fluorescence of four IR-GFP devices at 20h. 5, 3, 3/5, N represent for IR-3-GFP (BBa_K658017), IR-5-GFP (BBa_K658018), IR-3/5-GFP (BBa_K658019) and IR-GFP (BBa_K658016) respectively.
Figure 4, figure 5 and figure 6 illustrate that mutated promoters lux pR-3 (BBa_K658006) and lux R-5 (BBa_K658007) dramatically increased the fluorescence intensity at steady state compared with wild type promoter lux pR (R0062), while mutated promoter lux pR-3/5 (BBa_K658008) gave an even weaker expression of GFP than promoter lux pR (R0062). It might be explained that the mutagenesis at position 3 and position 5 of the sequence of lux pR (R0062) changed the binding strength between promoter lux pR and protein luxR.
Method
1.Isolation of Plasmid
Using the Procedure for GenEluteTM Plasmid Miniprep Kit
- Collect 1-5 mL bacterium fluid in 1.5 mL centrifuge tube,and centrifuge fluid at 12000 r/min
- Resuspend cells. Discard the supernatant and completely resuspend the bacterial pellet with 250 µl of the Resuspension Solution
- Lyse cells. Lyse the resuspended cells by adding 250 µl of the Lysis Solution
- Neutralize. Precipitate the cell debris by adding 350 µl of the Neutralization/Binding Solution, and centrifuge fluid at 12000r/min
- Load cleared lysate. Transfer the supernatant from step 4 to the spin column. Centrifuge at 12000r/min for 1 minute, and then discard the filtrate
- Optional wash. Add 500 µl of the Optional Wash Solution to the column. Centrifuge at 12000 r/min for 1 minute. Discard the filtrate
- Wash column. Add 500 µl of the diluted Wash Solution to the column. Centrifuge at 12000r/min for 1 minute.
- Elute DNA. Transfer the column to a new collection tube. Add 50~100 µl of Eluent Solution to the column. Centrifuge
at 12000 r/min for 1 minute. The DNA is now present in the filtrate and is ready for immediate use or storage at -20℃
2.Reaction system of restriction endonuclease
Figure 7 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
System1、2、3 and 4 are used for Standard BioBrick Assembly .System 5 and 6 are used for Restriction analysis. Digestion of sample: at least 500 ng DNA / 10 µL volume. Digest for 4 h at 37 °C, afterwards inactivated by adding 10x loading buffer and standing for 10 min at room temperature.
3. Standard BioBrick Assembly
- Digestion of insert: 2 μg~5 μg DNA / 100 µL volume, 10x H buffer, EcoRI, SpeI. Digestion and inactivation. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel, try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector: 2 μg~5 μg DNA / 100 µL volume, 10 x M buffer, EcoRI, XbaI. Digestion and inactivation. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel, try to avoid staining or exposure to ultraviolet light of the insert.
4. Suffix Insertion
- Digestion of insert: 2 μg~5 μg DNA / 100 µL volume, 10x M buffer, XbaI, PstI. Digestion and inactivation. Clean up the insert.
- Digestion of vector : 2 μg~5 μg DNA / 100 µL volume, 10x H buffer, SpeI, PstI. Digestion and inactivation. Clean up the vector.
5. Ligation
- After digestion and clean-up, the next step is ligation. Overnight ligation at 16°C. Table 2 is the system of ligation.
6. Transformation
- Preparation of competent E.coli cells
- Add 10 µL plasmid to 100 µl competent cells in centrifuge tube
- Store tube on ice for 20-30 minutes
- Water bath for 90s at 42℃
- Put the tube on ice for 1-2min
- Add 790 µL LB,and cultivation for 1h at 37 ℃,then plate on selective LB-Medium.
7. Restriction analysis
- Pick one colony with a sterile tip and cultivation in 20ml LB for overnight at 37 ℃
- Isolation of Plasmid
- Digest BioBrick,the system of Restriction analysis refer to table1
- Gel electrophoresis:add 2 µL loading buffer to digestion mixture. An agarose concentration is 1 %.
8. PCR -RBS1.0-luxR-TT-lux pR-
Design Primers using the primer 5
Primer:
2621-F:5'-GCTCTAGAGAAAGAGGAGAAATACT-3'
2621-R:5'-AAAACTGCAGCGGCCGCTACTA-3'
FIGURE 8 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
FIGURE 9 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
9. Cell growth
- 100µL suspension was inoculated from a Glycerin tube into 20ml fresh LB and incubated overnight at 37℃ and 250 r.p.m.
- 100µL suspension was inoculated again from step1 into 50ml fresh LB and incubated at 37℃ and 250r.p.m
- IPTG was added when A600≈0.6-0.8
- 1 ml suspension was taken on every sample taken time. 3 samples were taken in each time.
- Diluted each sample to 10-6(Sometimes 10-5),and then plate on selective LB-Medium.
- After 12h, count the number of CFU on the plate on different time point and then draw the cell growth curve.
FIGURE 10 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
10. Determining fluorescence intensity
- Add IPTG when A600 0.6~0.8.
- Cool the culture 10 minutes on ice.
- Centrifuge at 6000rpm. Wash it with pre-cooled PBS buffer.
- Use fluorescence spectrophotometer tomeasure the fluorescenceof GFP:
- Before measuring, dilute the bacteria with PBS buffer so that it can be within the measuring #range. Set excitation wavelength 491 nm, emission wavelength 511 nm.
- Transfer the measured bacteria in a new centrifuge tube and measure the OD of the bacteria.
11. Site Directed Mutagenesis
Figure 11 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
figure 12 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
•Digest the template plasmid by adding 1 µL of DpnI and incubate for 1-2 h
•Transform 10 µL of t PCR product into competent E. coli cells
•Screen the transformants using restiction digest and sequencing
12. Error prone PCR
figure 13 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
figure 14 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 718
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1874
Illegal BsaI.rc site found at 2601