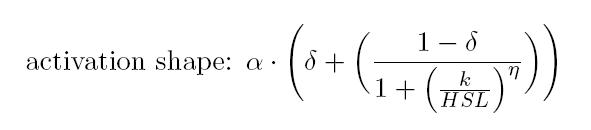Difference between revisions of "Part:BBa T9002:Experience"
Elena moreno (Talk | contribs) |
|||
| Line 115: | Line 115: | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
We used this construction as a model for a GFP-based characterization method. We tried different concentrations, that turned to be too high, for it seems that the media was quite saturated. | We used this construction as a model for a GFP-based characterization method. We tried different concentrations, that turned to be too high, for it seems that the media was quite saturated. | ||
| + | |||
| + | <nowiki> | ||
Fluor 0,4 0,2 0,02 0,002 | Fluor 0,4 0,2 0,02 0,002 | ||
| Line 183: | Line 185: | ||
180 578087,9652 602881,7454 556724,7688 578030,5195 | 180 578087,9652 602881,7454 556724,7688 578030,5195 | ||
| − | + | </nowiki> | |
| + | |||
| + | [[Image:GFPgraph.png]] | ||
<!-- DON'T DELETE --><partinfo>BBa_T9002 EndReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_T9002 EndReviews</partinfo> | ||
Revision as of 14:08, 1 October 2011
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_T9002
User Reviews
UNIQ643d87a984f5f38f-partinfo-00000000-QINU
2009 DNA Distribution quality control
The UNIPV-Pavia iGEM team sequenced T9002 part and found that it was completely confirmed, while iGEM QC results classified it as "inconsistent". DNA was resuspended from well 9A, kit plate 2, transformed in TOP10 E. coli and amplified inoculating a single colony from the grown LB agar plate in LB medium. Finally DNA has been miniprepped from the grown culture and sent to a BMR Genomics (Padova, Italy) for sequencing.
Experimental measurements
The UNIPV-Pavia iGEM team tested T9002 BioBrick in several working conditions. Results are reported in BBa_F2620 Experience page.
The Brown iGEM team conducted tests on this part in the summer of 2007. The results are depicted in the graphs below.
The first graph indicates that until a critical point is reached, increasing AHL concentration does increase GFP output. This is most easily noticeable between the concentrations of 20 nM, after which point the amount of GFP produced by cells begins to decrease. This may be due to one of two things: AHL quenches the signal from GFP, or too much AHL disrupts the cell's functions in a way that either kills it or prevents it from making as much GFP. This second hypothesis is partially confirmed by the second graph, which shows that adding more than 20 nM AHL causes a decline in cell density. On each graph, the different colored lines represent different time points after AHL was added to the cells. They are 4 hours, 5 hours, etc.
|
•••••
Antiquity |
This review comes from the old result system and indicates that this part worked in some test. |
UNIQ643d87a984f5f38f-partinfo-00000003-QINU
UNIQ643d87a984f5f38f-partinfo-00000004-QINU
|
•••••
UNIPV-Pavia iGEM 2011 |
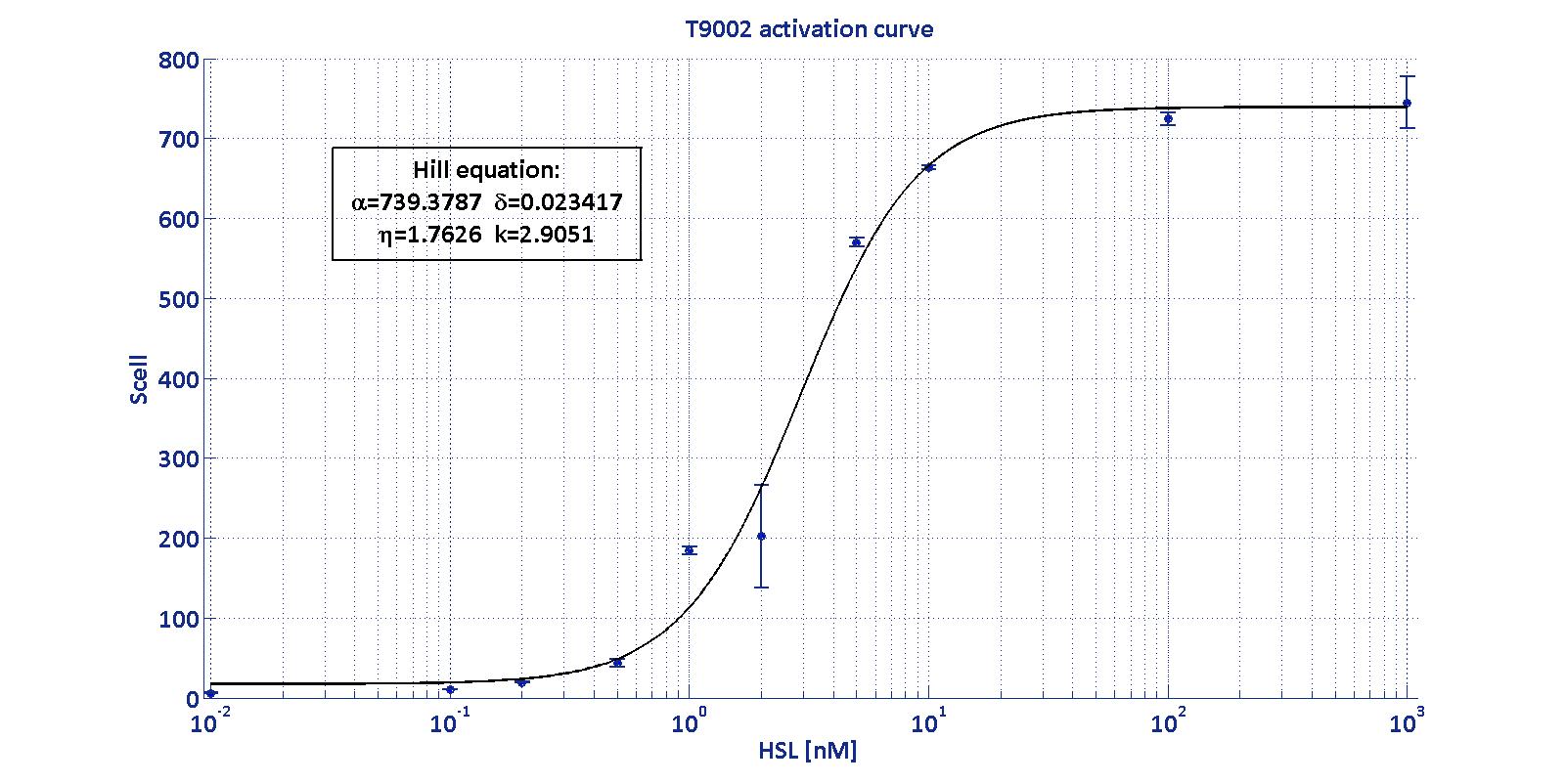
BioBrick BBa_T9002 is an HSL biosensor, which provides a non linear relationship between HSL input and Scell output. More precisely, the characteristic sigmoidal curve requires synthetic parameters for its accurate identification. These are the minimum and maximum values, the swtich point (i.e., the curve inflection point), and the upper and lower boundaries of linearity. This biosensor revealed greatly reliable, providing measurement repeatability and minimal experimental noise. Referring to its activation formula, the calibration curve is shown below.
In order to determine the threshold sensitivity of T9002 biosensor, experiments were performed with several HSL inductions minimally interspaced in the region of low detectability. Hypothesizing that the inducer is 1:20 diluted (as for all of our tests), the minimum detectable HSL concentration is 3 nM.
This biosensor was used to measure HSL concentration for parts producing or degrading this signalling molecule. For each of these experiments, a calibration curve of the biosensor was built, inducing it with known HSL concentrations, evaluating for each of them the Scell signal and finally estimating the Hill curve parameters. Once identified the parameters, the unknown concentration of a sample can be evaluated from its Scell (provided that it has a value included in the linear zone of the biosensor), as shown below: It is necessary, then, to multiply the measurement for the dilution factor used (in our experiments it was 20). |
|
••
|
We used this construction as a model for a GFP-based characterization method. We tried different concentrations, that turned to be too high, for it seems that the media was quite saturated. Fluor 0,4 0,2 0,02 0,002 0 3985 3841 4047 2632 0 3724 4055 3987 2505 30 19702 18805 21706 12111 30 19268 18227 20908 13187 60 33828 33142 37363 25733 60 35798 36573 42138 26591 90 88975 93532 85121 70231 90 78448 76621 59010 60932 120 134015 130912 139983 109002 120 118198 116762 143763 96888 150 201471 214457 223549 192318 150 200439 212693 221668 184846 180 235361 235361 235361 235361 180 235361 235361 235361 235361 Abs 0,4 0,2 0,02 0,002 0,197 0,175 0,177 0,17 0,14 0,196 0,179 0,167 0,149 0,132 0,225 0,218 0,175 0,196 0,17 0,238 0,21 0,179 0,196 0,163 0,204 0,191 0,195 0,212 0,207 0,23 0,224 0,216 0,234 0,23 0,321 0,3 0,304 0,287 0,275 0,304 0,253 0,238 0,172 0,247 0,349 0,295 0,275 0,299 0,285 0,275 0,25 0,225 0,305 0,246 0,36 0,338 0,365 0,359 0,373 0,339 0,328 0,359 0,367 0,362 0,439 0,38 0,376 0,398 0,37 0,357 0,373 0,388 0,411 0,422 Fluo/Abs 0,4 0,2 0,02 0,002 20228,4264 21948,57143 23805,88235 18800 19000 22653,63128 26758,38926 18977,27273 87564,44444 86261,46789 110744,898 71241,17647 80957,98319 86795,2381 106673,4694 80901,84049 165823,5294 173518,3246 176240,566 124314,0097 155643,4783 163272,3214 180076,9231 115613,0435 277180,6854 311773,3333 296588,8502 255385,4545 258052,6316 302849,8024 343081,3953 246688,2591 383997,1347 443769,4915 468170,5686 382463,1579 429810,9091 467048 471354,0984 393853,6585 559641,6667 634488,1657 622699,1643 515597,8552 591265,4867 648454,2683 604000 510624,3094 536129,8405 619371,0526 591359,2965 636110,8108 659274,5098 630994,6381 572654,5012 557727,4882 Average 0,4 0,2 0,02 0,002 19614,2132 22301,10136 25282,13581 18888,63636 84261,21382 86528,35299 108709,1837 76071,50848 160733,5038 168395,323 178158,7446 119963,5266 267616,6585 307311,5679 319835,1228 251036,8568 406904,0219 455408,7458 469762,3335 388158,4082 575453,5767 641471,217 613349,5822 513111,0823 597702,1752 625182,8454 582006,8988 596919,1495 Average variation of the Fluo/Abs ratio Time (min) 0,4 0,2 0,02 0,002 0 0,00319797 0,001356744 0,005807343 0,006363636 30 64647,00382 64227,25299 83427,05367 57182,87848 60 141119,2938 146094,223 152876,6146 101074,8966 90 248002,4485 285010,4679 294552,9928 232148,2268 120 387289,8119 433107,6458 444480,2035 369269,7782 150 555839,3667 619170,117 588067,4522 494222,4523 180 578087,9652 602881,7454 556724,7688 578030,5195
|