Difference between revisions of "Part:BBa K546005"
RubenvanHeck (Talk | contribs) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K546005 short</partinfo> | <partinfo>BBa_K546005 short</partinfo> | ||
| − | |||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | + | [[Image:Visualization_LuxR_LuxI_GFP.png|520px|thumb|A schematic representation of the BioBrick sub parts' interaction.]] | |
| + | This device has three protein generators: | ||
| + | lux pl-RBS-C0062-B0015 produces LuxR. LuxR forms a complex with the quorum sensing molecule AHL and this complex subsequently increases the transcriptional rate of the lux pR promoter while also decreasing the transcriptional rate of the lux pL promoter. Both of the other generators are under control of this lux pR promoter. | ||
| − | + | The second generator ‘luxpR-RBS-J04031-B0015’has a low basal expression of the GFP, which can - of course - be monitored. | |
| + | The third generator produces luxI, which produces the quorum sensing molecule AHL. In combination with LuxR, AHL thus increases the production of AHL and GFP. | ||
===Safety Aspects=== | ===Safety Aspects=== | ||
| Line 20: | Line 21: | ||
<span class='h3bb'>'''Sequence and Features'''</span> | <span class='h3bb'>'''Sequence and Features'''</span> | ||
<partinfo>BBa_K546005 SequenceAndFeatures</partinfo> | <partinfo>BBa_K546005 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | |||
| + | ==Experimental data== | ||
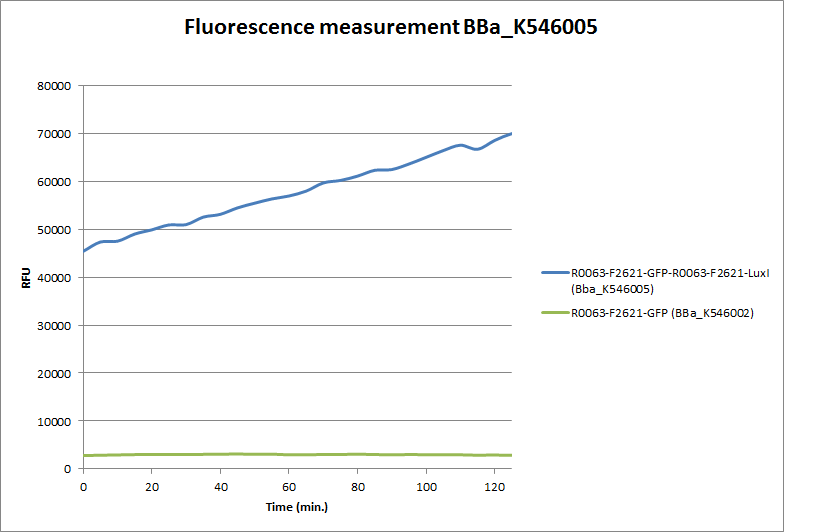
| + | This part was tested by measuring overnight <I>E. coli</I> cultures transformed with high copy plasmid pSB1A2 containing this insert. Fluorescence was measured in a Molecular Devices Spectramax M2 spectrophotometer, exciting the samples at 485 nm and detecting at 510 nm. 200 µL samples were analyzed in an opaque 96-wells plate. The GFP expression was monitored for 2 hours, with BBa_K546002 transformants (high copy, pSB1A2) as negative control. | ||
| + | |||
| + | The outcome is shown below. R0063-F2621 refers to the repaired version of F2621, submitted as [https://parts.igem.org/Part:BBa_K546003 K546003]. | ||
| + | |||
| + | [[Image:Fluorescence_measurement_BBa_K546005-BBa_K546002correct.png]] | ||
| + | |||
| + | GFP expression rapidly increases over time, indicating a functional positive feedback loop. | ||
Latest revision as of 02:43, 22 September 2011
Lux pL controlled LuxR + lux pR autoinducing LuxI (lva tag) + lux pR controlled GFP(lva tag).
Usage and Biology
This device has three protein generators: lux pl-RBS-C0062-B0015 produces LuxR. LuxR forms a complex with the quorum sensing molecule AHL and this complex subsequently increases the transcriptional rate of the lux pR promoter while also decreasing the transcriptional rate of the lux pL promoter. Both of the other generators are under control of this lux pR promoter.
The second generator ‘luxpR-RBS-J04031-B0015’has a low basal expression of the GFP, which can - of course - be monitored.
The third generator produces luxI, which produces the quorum sensing molecule AHL. In combination with LuxR, AHL thus increases the production of AHL and GFP.
Safety Aspects
This BioBrick part produces an autoinducer that might interact with [http://2011.igem.org/Team:Wageningen_UR/Safety/One#Risk_Identification_of_BioBrick_System_Inside_the_Cell_Chassis some pathogens].
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 3909
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1101
Illegal BsaI.rc site found at 1828
Illegal BsaI.rc site found at 2086
Illegal BsaI.rc site found at 3189
Experimental data
This part was tested by measuring overnight E. coli cultures transformed with high copy plasmid pSB1A2 containing this insert. Fluorescence was measured in a Molecular Devices Spectramax M2 spectrophotometer, exciting the samples at 485 nm and detecting at 510 nm. 200 µL samples were analyzed in an opaque 96-wells plate. The GFP expression was monitored for 2 hours, with BBa_K546002 transformants (high copy, pSB1A2) as negative control.
The outcome is shown below. R0063-F2621 refers to the repaired version of F2621, submitted as K546003.
GFP expression rapidly increases over time, indicating a functional positive feedback loop.