Part:BBa_K2770006
GLYR1 Generator for S. cerevisiae
Usage and Biology
Glycolic acid is a commonly used component in cosmetics and in medicinal applications. It is a simple α-hydroxy acid with a molecular weight of 76 g/mol [1]. Through the functional hydroxy- and acid-groups the molecule is highly soluble in water. This property makes glycolic acid polymers attractive for many applications in industry, e.g. in the textile, leather, oil, and gas industry[2]. The polymer of glycolic acid has an excellent gas barrier property which is an optimal base for e.g. packaging materials[3]. In combination with lactic acid, the created polymer PLGA is used as drug delivery system in medical applications[4]. Every year the demand for this C2-body increases. At the moment the production depends on toxic and environmentally unfriendly chemicals[5], pointing to the need of an alternative production method.
Glycolic acid in cells is a by-product of the glyoxylate cycle. Modifications of the glyoxylate cycle through deletion and overexpression of certain genes make the production of glycolic acid in S. cerevisiae possible. Another important genetic modification is the introduction of a glyoxylate reductase, which converts glyoxylate into glycolic acid by reducing the aldehyde group in a NADPH-dependent reaction. S. cerevisiae contains the naturally occurring gene GOR1, but this gene is not expressed[6] under normal growth conditions and the encoded glyoxylate reductase shows moderate affinity to the substrate glyoxylate. Therefore, we used AtGLYR1 from Arabidopsis thaliana instead. Previous studies reported an enhanced affinity of AtGLYR1 to glyoxylate[7] and also enhanced activity [5].
Figure 1: Structure of the NADPH-dependent glyoxylate reductase (AtGLYR1) with a molecular weight of 30.7 kDa.
To learn more about AtGLYR1 and its part in our project, please visit our [http://2018.igem.org/Team:TU_Darmstadt/Project/Glycolic_acid/S_cerevisiae wiki].
Mechanism
The glyoxylate reductase converts glyoxylate into glycolic acid by reducing the aldehyde group in a NADPH-dependent reaction.

Figure 2: Reaction mechanism of NADPH-dependent conversion of glyoxylate into glycolic acid performed by AtGLYR1.
Methods
Expression
The AtGLYR1 gene was synthesized by IDT. We inserted the gene into BioBrick vector pSB1C3 vector and verified the DNA sequence by sanger sequencing (Eurofins Genomics). The gene AtGLYR1 was expressed under control of constitutive ADH promotor in pAT423 in yeast. To examine the successful expression, a western blot was performed.

Figure 3:pSB1C3 plasmid including AtGLYR1.

Figure 4:pAT423 plasmid including expression cassette of AtGLYR1.
SDS-PAGE and Western Blot
To verify that AtGLYR1 was heterologously produced, a SDS-PAGE was performed, followed by a western blot. The resulting bands were compared to the expected protein size of 32.9 kDa.
Purification
After expression of AtGLYR1 in S. cerevisiae, a GE Healthcare ÄKTA Pure machine was used to purify the desired Strep-tagged enzyme.
Enzyme assays
The assay for the glyoxylate reductase AtGLYR1 is based on the different absorption maxima of NADPH (absorption maxima at 260 and 340 nm) and NADP+ (maximum at 260 nm). During the reaction, the enzyme uses NADPH as a cofactor. NADPH is converted into NADP+, which leads to a decrease of absorption at 340 nm. By measuring the absorption at 340 nm over time, it is possible to quantify the enzyme activity and infer glycolic acid production. To calculate the NADPH conversion rate, a calibration curve was created by measuring the absorption of different NADPH concentrations.

Figure 5:Reaction mechanism of NADPH-dependent conversion of glyoxylate into glycolic acid performed by AtGLYR1.
HPLC analysis
To detect the produced monomer (glycolic acid), as well as its precursors, high-performance liquid chromatography (HPLC) was utilized. An organic acid separation column as stationary phase and sulfuric acid as mobile phase were used. This allowed the separation of glycolic acid, isocitrate and glyoxylate. Signals were recorded by a refractive index detector.
Results
Cloning and Expression
To produce glycolic acid in S. cerevisiae, a glyoxylate reductase from Arabidopsis thaliana is needed to be expressed. The native glyoxylate reductase GOR1 does not have an affinity to glyoxylate as high as AtGLYR1 from A. thaliana. A S. cerevisiae codon optimized version of the sequence coding for AtGLYR1 containing a Strep-tag was ordered from IDT. The sequence was inserted into a shuttle vector (pAT423), which can be used for gene expression in S. cerevisiae, as well as in E. coli. The cloning was done using the Gibson assembly method. The accuracy of the cloning was confirmed by sequencing.
To test whether AtGLYR1 was produced in S. cerevisiae, we performed a western blot. We tested three colonies which were grown on selective medium after transformation with AtGLYR1 x pAT423. As a negative control, untransformed CEN.PK-1C cells were used. The resulting western blot is shown in Figure 6. AtGLYR1 was designed to contain a Strep-tag. Therefore, an anti-Strep antibody was used to detect the tagged protein with an expected molecular mass of 31.9 kDa.

Figure 6:Western blot for AtGLYR1. Three colonies were screened for the translation of AtGLYR1 in S. cerevisiae. Thermo Scientific PageRuler Prestained Protein Ladder was used as protein standart. 1-3 = CEN.PK 1C was transformed using pAT423xAtGLYR1; - = Negative control of untransformed CEN.PK 1C.
The western blot shows the results of three colonies, which were screened for the production of AtGLYR1. Colony 1 and 3 produced AtGLYR1. Colony 2 shows no band at the expected height. A corresponding positive colony was used for further experimental procedures. After gene expression for up to 22h, the protein was purified via StrepTactin columns using an ÄKTA system.
Enzyme assay
The purified enzyme was subjected to a determination of its biological activity. The assay was based on the oxidization of NADPH. While the purified AtGLYR1 oxidizes NADPH, glyoxylate is being reduced to glycolic acid. The conversion of NADPH to NADP+ can be recognized through a decrease in absorption at 340 nm. Since the conversion of NADPH comes along with the reduction of glyoxylate, this assay can proof the activity of the enzyme and the possible in vitro production of glycolic acid. For the calibration curve, the absorption of different concentrations of NADPH was measured and used to calculate the substrate turnover rate (figure 7). The purified AtGLYR1 was assayed at 30 °C (figure 8).

Figure 7:Calibration curve for AtGLYR1 enzyme assay. Different NADPH concentrations (1 mM, 0.5 mM, 0.25 mM, 0.125 mM and 0 mM) were measured and plotted against their average absorption. All samples were measured in triplicates.

Figure 8:NADPH dependent enzyme assay of AtGLYR1 at 30 °C. The graph shows the average absorption at 340 nm in correlation to the time (min). For the enzyme assay 2.7 ng/µL were used, positive (NADP+) and negative control (NADPH) were included. All samples were measured in triplicates.
The average absorption of both, the positive and negative controls, stays constant over the time. After about 30 minutes of reaction time, the curve almost reaches the absoprtion level of the positive control, consisting of pure NADP+. It can be assumed that the decrease of the average absorption in the enzyme containing sample is due to the functionality and activity of AtGLYR1, which oxidizes NADPH. The concentration of NADPH at the start of the assay was the same as for the negative control. Even though the enzyme is added to the reaction tube as the last component, the enzyme starts to work right before the first measurement point is recorded, so that the initial value was only 0.9 instead of 1.2.
The substrate conversion values were recorded in the linear range of the particular curve in a time frame of 4 minutes. Thus, 0.53 µM/s was the calculated substrat conversion rate of AtGLYR1 at 30 °C.
Next, a Student’s t-test was performed to see whether or not these results were significant. For this purpose, five data sets (0, 5, 10, 20 and 30 minutes) were chosen (figure 9).

Figure 9:Enzyme assay absorption for 0, 5, 10, 20 and 30 minutes with standard error. n=3
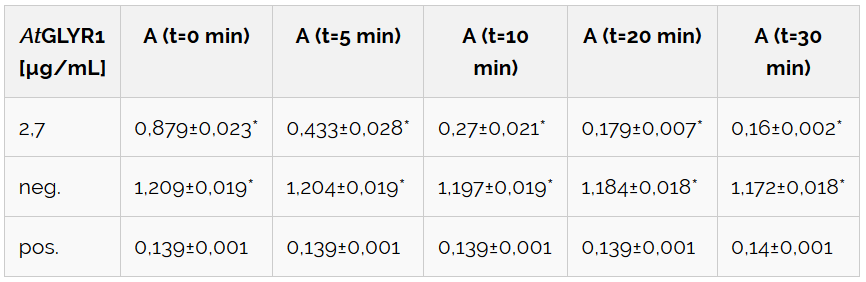
Table 1 includes the values for figure 9. It also shows the NADPH-absorption at chosen times, as well as the associated standard error.
Table 1: Calculated significance of AtGLYR1 data sets for 0, 5, 10, 20 and 30 minutes at 30 °C. The table also shows the NADPH-absorption, as well as the standard error. A= Absorption, *= Significance (p-value < 0.05), n= 3
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 726
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 822
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
- ↑ Fermentation process for producing glycolic acid, 18/10/14 [1].
- ↑ Glycolic Acid [2].
- ↑ Engineering Escherichia coli for glycolic acid production from D-xylose through the Dahms pathway and glyoxylate bypass. [3].
- ↑ The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems--a review. [4].
- ↑ 5.0 5.1 Glycolic acid production in the engineered yeasts Saccharomyces cerevisiae and Kluyveromyces lactis [5].
- ↑ The ORF YNL274c (GOR1) codes for glyoxylate reductase in Saccharomyces cerevisiae [6].
- ↑ Characteristics of an Arabidopsis glyoxylate reductase: general biochemical properties and substrate specificity for the recombinant protein, and developmental expression and implications for glyoxylate and succinic semialdehyde metabolism in planta [http://www.nrcresearchpress.com/doi/abs/10.1139/B07-081#.W8CLm_ZCTIV].
| None |
