Help:Terminators/Design
There are two ways to design new terminator genetic parts. You can build parts based on transcriptional terminators from natural genomes, like that of E. coli K-12. (There are at least 135 reported terminators in the E. coli K-12 genome d-Aubenton-Carafa, Lesnik.) Or alternatively, you can design synthetic transcriptional terminators de novo using naturally occurring terminator sequences as a basis. Regardless of which approach you choose, there are a few basic design guidelines for transcriptional terminators that you should know. We outline those here.
General considerations for terminator design
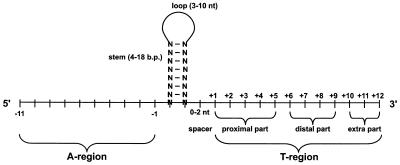
- The two most common components of a forward rho-independent transcriptional terminator in E. coli are a stem loop that is rich in G-C base pairs, followed by a poly(T) tract (T region in the figure). The stem loop and poly(T) tract are (usually) necessary and sufficient for transcriptional termination Christie, d-Aubenton-Carafa.
- Although the stem loop and poly(T) tract constitute a minimal transcriptional terminator, sequences both upstream from the stem loop and downstream of the poly(T) tract can significantly influence termination efficiency Reynolds. Thus, terminator designs based on naturally occurring sequences are best verified by experimental characterization.
- Bidirectional terminators should also include a poly(A) tract prior to the stem loop, so that the terminator can function in both directions d-Aubenton-Carafa.
- When designing new terminator parts from naturally occurring sequences, keep in mind that even simple mutations to the stem loop, such as inversion of a G-C base pair to C-G can cause a dramatic decrease in termination efficiency Cheng. Conversely, in some cases, mutations disrupting some of stem loop structure will have little impact on termination efficiency Cheng. Thus, again the function of transcriptional terminators are best verified by experimental characterization.
Sequence constraints
To maximize termination efficiency
- The stem length should be 8-9 base pairs, primarily G-C in content. Longer or shorter stem loops tend to have reduced termination efficiency Wilson.
- The loop should be 4-8 residues, although 55% of the terminator stem loops are 4 residues and the most abundant loop sequences are
UUCGandGAAAWilson, d-Aubenton-Carafa. - Within the above limits, termination efficiency can be increased by increasing the stability of the hairpin Wilson.
- Stem loops should close with a C-G base pair d-Aubenton-Carafa.
- The poly(T) tract should be 6-8 residues in length and located just downstream of the stem loop Wilson.
- Termination will typically occur at U7 or U8 of the mRNA Wilson.
Of 148 experimentally verified and putative transcriptional terminators d-Aubenton-Carafa
- 85% of terminator stem loops are 3-5 residues.
- 75% of the stems are 7(+/-2) base pairs in length.
- 41% have more than 80% C-G or G-C base pairs.
- 59% of terminator stem loops close with a C-G base pair.
- 80% of terminator stem loops close with 3 C-G/G-C base pairs.
Note: the termination efficiency of terminators is very sequence-, and to a lesser extent, context-dependent. Therefore, the above rules are simply guidelines and may not in fact result in functional transcriptional terminators.
References
<biblio>
- d-Aubenton-Carafa pmid=1702475
- Cheng pmid=1835546
- Christie pmid=7027254
- Lesnik pmid=11522828
- Reynolds pmid=1372366
- Wilson pmid=7568019
</biblio>