Part:BBa_K3739015
PPO-his
The enzyme efficiently catalyzes the reaction of oxidizing dopaming to dopa quinone. His-tag was added to purify the protein.
Biology
The polyphenol oxidase we used is a kind of polyphenol oxidase from the Marine mussel itself, which regulates the mucin production of mussel. It can oxidase the dopamine on the side chain of mussel mucin to dopamine quinone, and its catalytic effect can be characterized by the change of viscosity characteristics of mussel mucin.
- Fig.1. the mechanism diagram of PPO
Usage
According to the biological characteristics of VnDX, we optimized the codon and added a 6His-tag to construct a sequence suitable for expression in VnDX. We hope that it can successfully express polyphenol oxidase in VnDX and reduce the viscosity characteristics of mussel mucin. The coding sequence of target gene was inserted into the expression vector, and the constructed plasmid was transformed into VnDX to verify its heterologous expression.
- Fig.2. Gene circuit diagram of PPO
Characterization
1. Identification
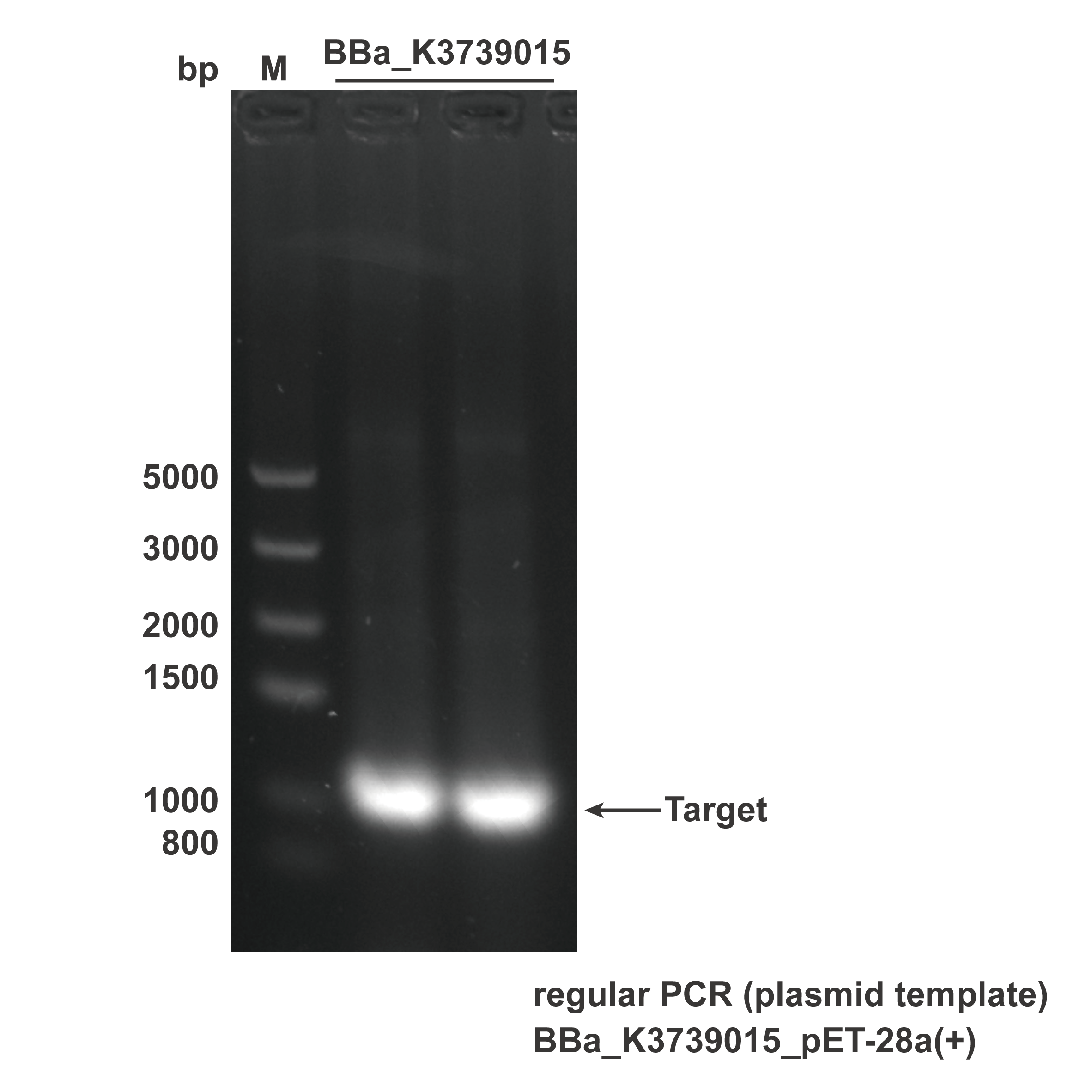
After we received the synthesized DNA sequence, PCR was conducted to verity the correctness of the plasmid, and the experimental results are shown in Fig.3.
- Fig.3. The result of regular PCR. Plasmid pET-28a(+).
2. Purification and Proof of the expression
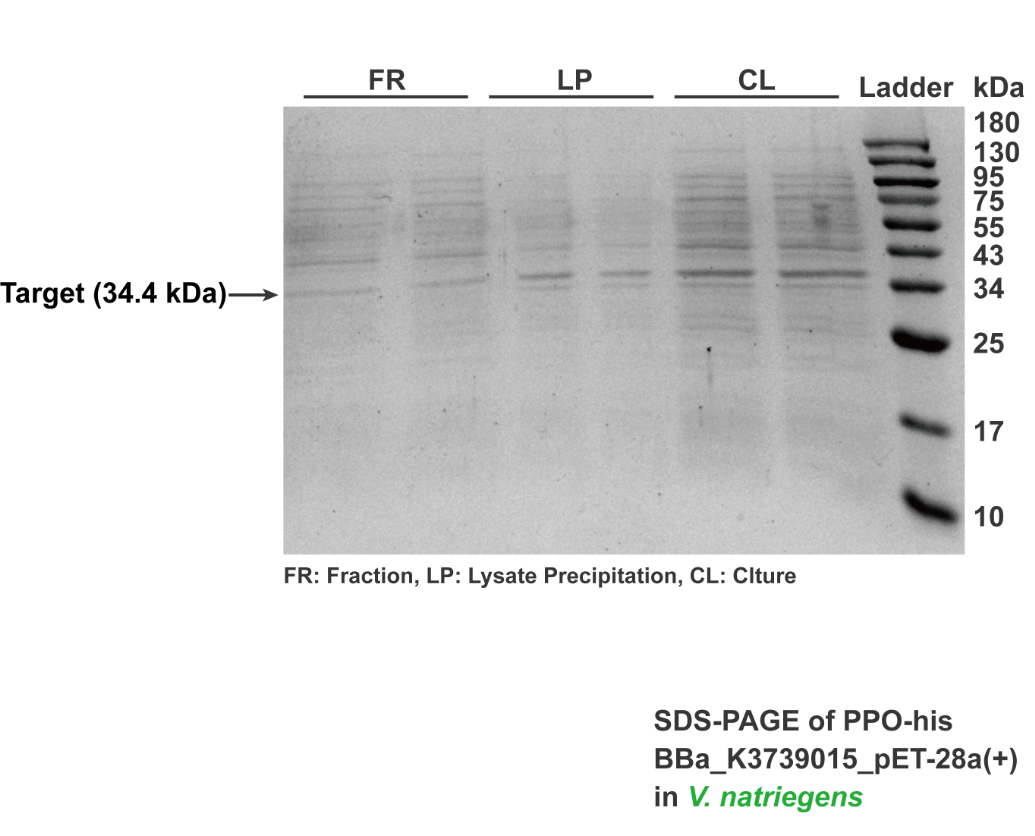
The mussel can adhere to a solid surface mainly through 3,4-dihydroxyphenylalanine (DOPA), the side chain in mucoprotein. We designed a genetic circuit to express polyphenol oxidase (PPO), which could degrade the DOPA. According to the literature, DOPA could be catalyzed by PPO to generate dopaquinone and significantly weaken mucoprotein viscosity. Thus, we express the PPO in Vibrio natriegens and characterization its enzyme activity, which is derived from Bacillus Megaterium (PDB ID: 3NM8). This PPO does not require a caddie protein for activity and possesses superior catalytic activity in the mixture of water and organic reagents. The brick of BBa_K3739015 has assembled into pET-28a(+) plasmid backbone with T7 promoter to express the PPO-his. After being verified by SDS-PAGE (Fig. 4), PPO was successfully expressed in E. coli BL21(DE3) and Vibrio natriegens.
3. Quantitative analysis of dopaquinone
The constructed plasmid was transformed into Vibrio natriegens through electroporation. Positive colonies were selected by kanamycin preliminarily and then verified by colony PCR and sequencing. Then, the colony with the corrected sequence was cultivated to express PPO. After ultrasonication broke and centrifugation, GE AKTA Prime Plus FPLC System was employed to purify the PPO from the supernatant. Purified protein was verified by electrophoresed on a sodium dodecyl sulfate (SDS)-10% (wt/vol) polyacrylamide gel, followed by Coomassie blue staining (Fig. 4).
Fig.4 SDS-PAGE analysis of PPO. Target bands (34.9kD kDa) can be observed at the position around 50 kDa.
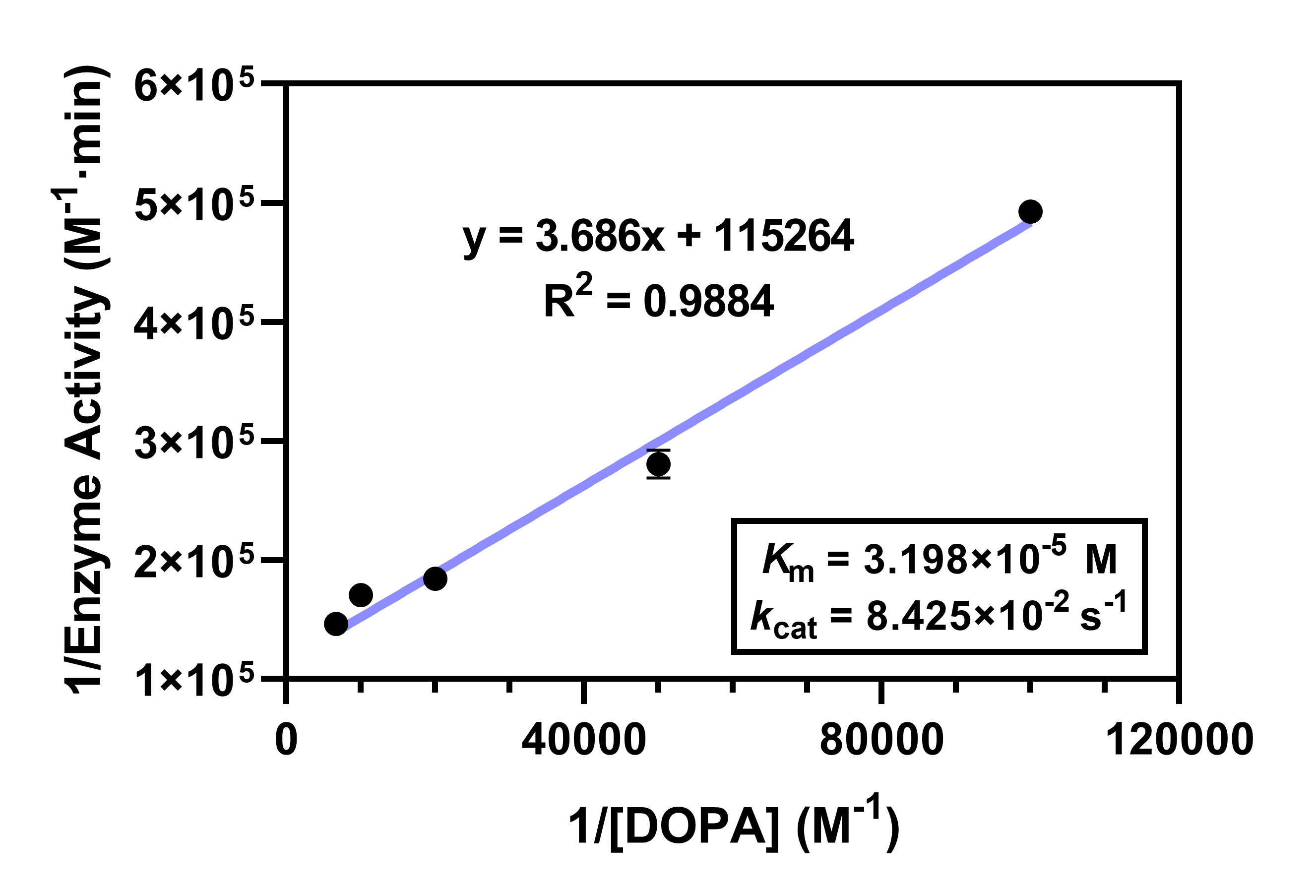
The time course of the absorbance of dopaquinone was measured at 475 nm to obtain the data to calculate the enzyme activity. Test system: sodium acetate buffer (170 μl), DOPA solution (20 μl, 50 mM) and PPO solution (10 μl) were added to the 96-well plate to measure the absorbance during the catalytic process through ECAN ® Infinite M200 Pro instrument. Parallel experiments were performed three times. Kinetics of enzyme catalysis The effects from different concentration of DOPA (0.01 mM, 0.02 mM, 0.05 mM, 0.1 mM and 0.15 mM) on the PPO catalytic activity were investigated with 0.1 M sodium acetate buffer (pH=6.0). And the catalytic kinetic parameters of PPO were also obtained through calculation.
- Fig.5 The relationship of 1/Enzyme activity and 1/concentration of DOPA.
Reference
1. Sendovski, M.; Kanteev, M.; Ben-Yosef, V. S.; Adir, N.; Fishman, A., First structures of an active bacterial tyrosinase reveal copper plasticity. J Mol Biol 2011, 405 (1), 227-37.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |